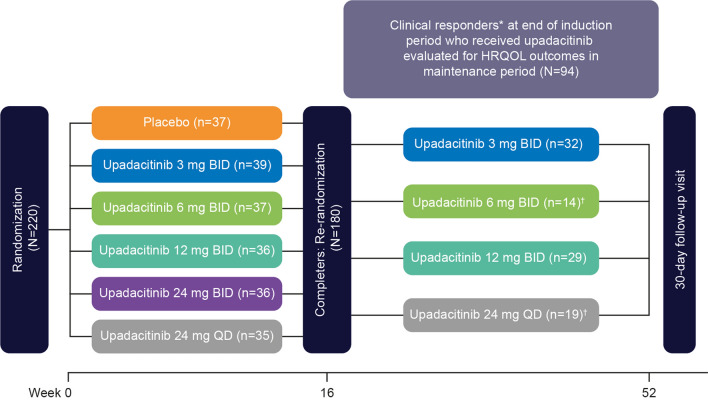
Fig. 1.
Study design. *Clinical responders defined as ≥ 30% decrease from baseline in average daily very soft/liquid stool frequency OR ≥ 30% decrease from baseline in average daily abdominal pain score, and neither worse than baseline. †Upadacitinib 6-mg BID dosage initiated and randomization for upadacitinib 24-mg QD dosage stopped with those currently enrolled at 24 mg QD continuing treatment per protocol amendment. BID twice daily; HRQOL health-related quality of life; QD once daily