FIGURE 2.
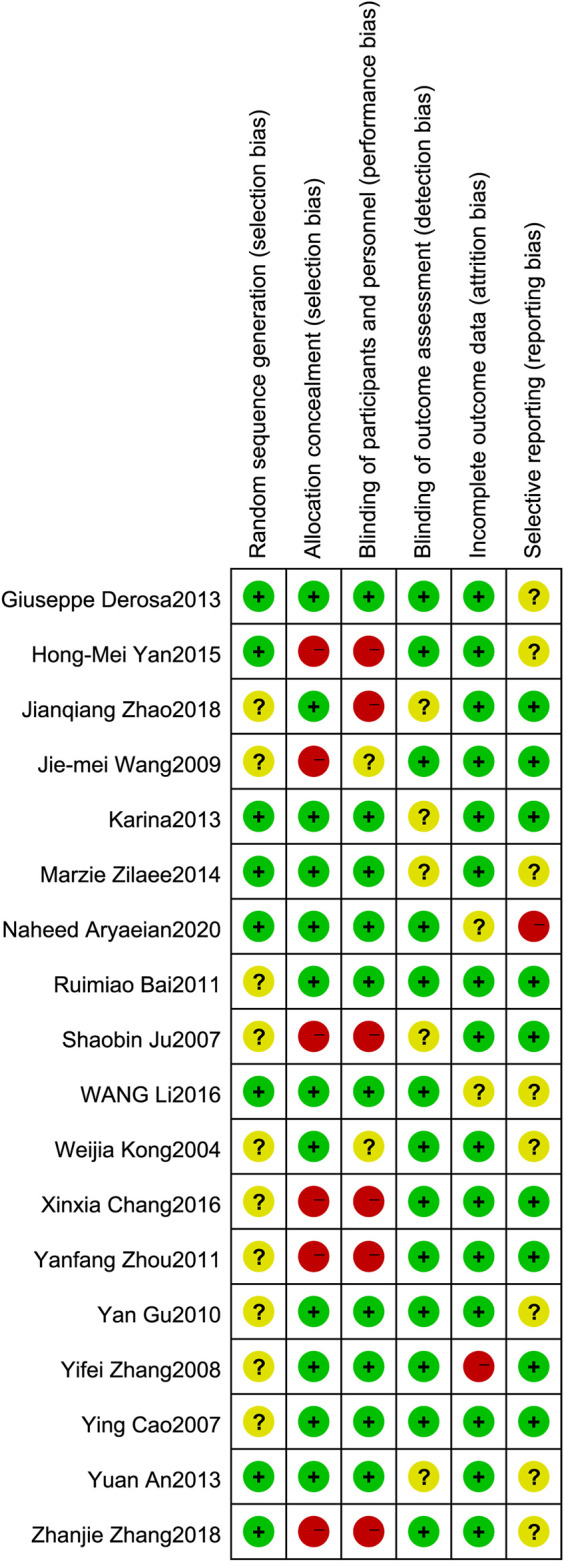
Risk of bias assessment. The details were related to the six domains that contained (1) random sequence generation (selection bias): the literature proclaims that the random sequence method is considered low risk. (2) Allocation concealment (selection bias): explain that allocation concealment or placebo-controlled experiments are considered low risk. (3) Blinding of participants and personnel (performance bias): the literature states that a double-blind study design is considered low risk. (4) Blinding of outcome assessment (detection bias): there is a return visit record or related explanation in the article that is considered low risk. (5) Incomplete outcome data (attrition bias): no outliers during the trial are considered low risk. (6) Selective reporting (reporting bias): no selective report treatment data are considered low risk.
