Figure 5.
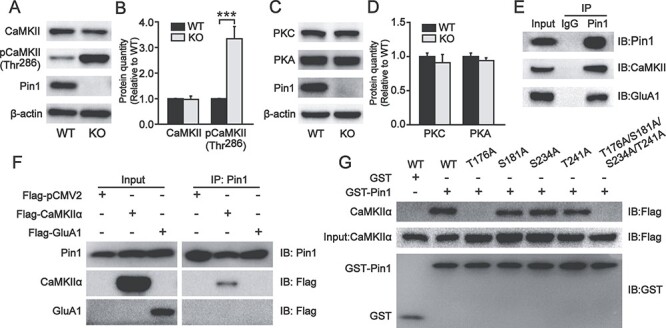
The expression of phosphorylated CaMKII Thr286 increased in the PFC of Pin1 KO mice, and Pin1 could interact with CaMKII and GluA1. (A, B) The expression of total CaMKII was unaltered in PFC slices of Pin1 KO mice (n = 6). However, phosphorylated CaMKII Thr286, which is a specific kinase for GluA1 Ser831, increased significantly (n = 6; ***P < 0.001). (C, D) These two kinases for GluA1, PKC, and PKA showed no change in Pin1 KO mice (n = 6). (E) Coimmunoprecipitation of endogenous Pin1 complexes from WT mouse PFC homogenates. Western blots were performed with anti-CaMKII and anti-GluA1 antibodies. Rabbit IgGs were used as a negative control. CaMKII and GluA1 can both be coprecipitated by endogenous Pin1 (n = 4). (F) Samples of HEK293T cells expressing CaMKIIα-Flag or GluA1-Flag were immunoprecipitated with anti-Pin1 antibody. Western blots were performed with anti-Flag antibody for CaMKIIα and GluA1 detection, showing that only CaMKIIα can be detected (n = 4). (G) GST-Pin1 pulldown from samples of HEK293T cells expressing CaMKIIα-Flag WT or mutants: 4 putative Pin1 binding sites Thr or Ser were mutated to Ala (T176A, S181A, S234A, T241A, and T176A/S181A/S234A/T241A). GST was used as a negative control. Pulled down CaMKIIα-Flag mutants were detected using an anti-Flag antibody. CaMKIIα, including the T176A mutant, could not be detected in GST-Pin1 pulldown assays (n = 4).
