Fig. 1.
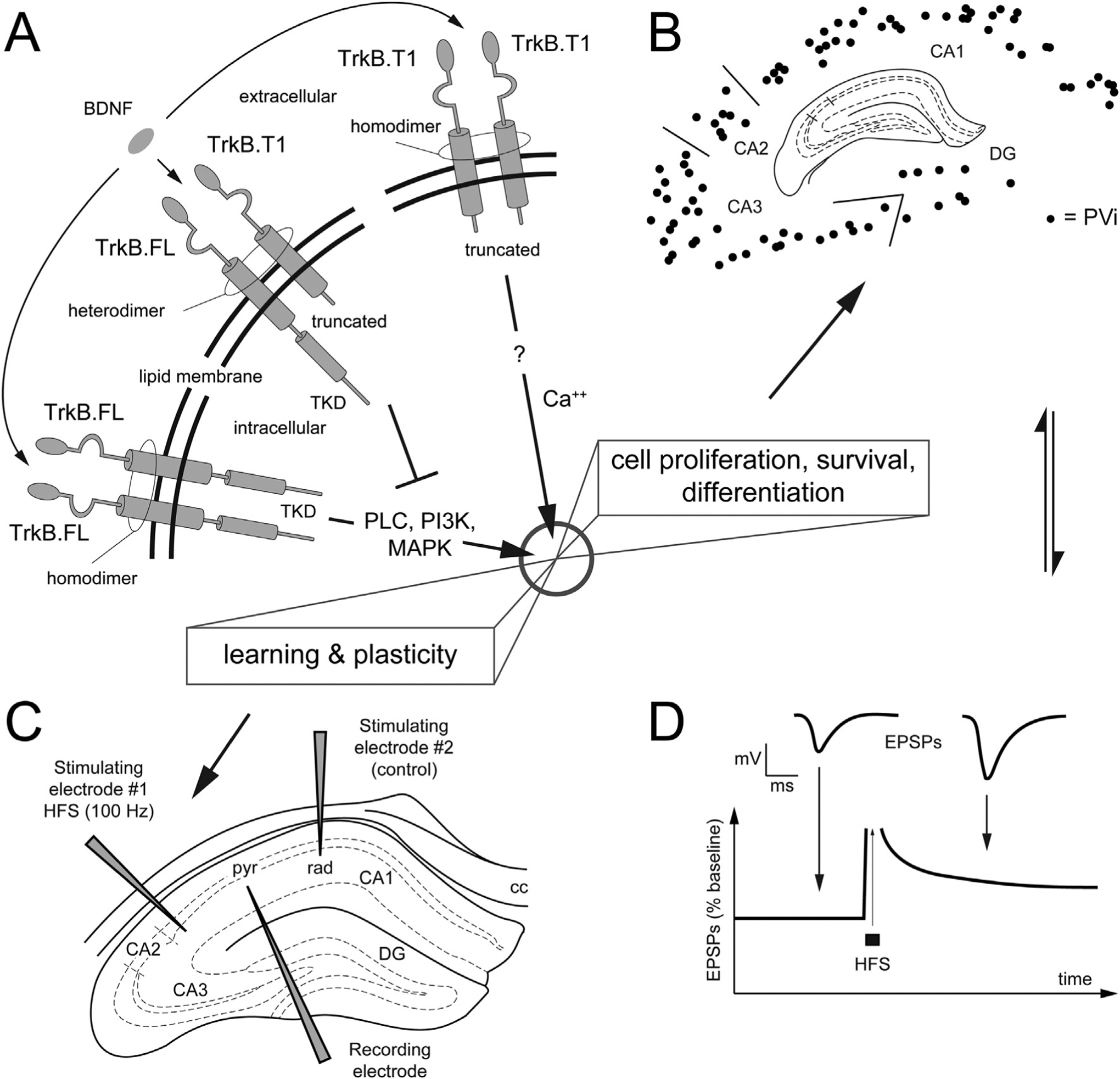
Molecular structure of TrkB isoforms and morphofunctional components investigated in the present work.
A. Schematic representation of the molecular structure of TrkB isoforms in the lipid membrane (full length = FL, truncated isoform T1). Extracellularly, the TrkB receptors are formed by a combination of Cysteine Rich Repeats, Leucine Rich Repeats and Immunoglobulin domains that is identical across isoforms. Intracellularly, the tyrosine kinase domain (TKD) is unique to the FL isoform. In the presence of brain-derived neurotrophic factor (BDNF), TrkB·FL homodimerization leads to receptor autophosphorylation at the TKD and downstream signaling (e.g., via the phosphorylation of PLC, PI3K, MAPK) to induce cell proliferation, survival/death, differentiation as well as plasticity. The TrkB.T1 isoform contains a short intracellular domain and lacks the TKD. In the presence of BDNF, T1 forms a heterotimer with FL, to exert its dominant negative inhibition (interrupted line in the figure) of FL-mediated signaling. In astrocytes, T1 is the only expressed isoform, where it controls the sequestration of BDNF. Note that while FL and T1 are the main isoforms, other isoforms exist, i.e., T2 e SHC (not shown).? = unknown intracellular signaling.
B, C, D. Morphofunctional components investigated in the present study: we focus our neuroanatomical analysis on a subpopulation of hippocampal inhibitory neurons that express parvalbumin (PV), the PV+ interneurons (PVi). B. Schematics of the charts PVi; our structural approach on PVi is complemented with electrophysiological investigation (C, D) aimed at comparing synaptic plasticity at the Schaffer’s collateral, via classic LTP experiments.
