Abstract
After 7-valent pneumococcal conjugate vaccine introduction in the United States in 2000, invasive pneumococcal disease (IPD) due to serotype 4 greatly decreased in children and adults. Starting in 2013, serotype 4 IPD incidence increased among adults within 3 of 10 Active Bacterial Core surveillance sites. Of 325 serotype 4 cases among adults in 2010–2018, 36% were persons experiencing homelessness (PEH); incidence of serotype 4 IPD among PEH was 100–300 times higher than in the general population within these 3 areas. Genome sequencing for isolates recovered 2015–2018 (n = 246), revealed that increases in serotype 4 IPD were driven by lineages ST10172, ST244, and ST695. Within each lineage, clusters of near-identical isolates indicated close temporal relatedness. Increases in serotype 4 IPD were limited to Colorado, California, and New Mexico, with highest increases among PEH, who were at increased risk for exposure to and infections caused by these strains.
Keywords: pneumococcal polysaccharide serotype 4, conjugate vaccines, persons experiencing homelessness
The past 2 decades have shown evidence of remarkable success of the first- and second- generation pneumococcal conjugate vaccines in greatly reducing invasive pneumococcal disease (IPD) [1, 2]. Although both vaccines have been used primarily to target infants and young children, large reductions in the burden of IPD caused by vaccine serotypes were observed in older individuals through indirect (herd) effects. The second generation pneumococcal 13-valent conjugate vaccine (PCV13) has further reduced IPD caused by the 7 serotypes in common with PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F) as well as additional serotypes in PCV13 (1, 3, 5, 6A, 7F, and 19A), with the exception of type 3 for which both direct and indirect effects of the vaccine have been minimal [2]. Serotype 4 IPD, targeted by both vaccines, showed steady declines in all age groups during the post-PCV7 years (2001–2009) [1] that continued into the early post-PCV13 years [2]. Following PCV7 introduction in 2000, there were dramatic decreases of IPD incidence caused by serotype 4 documented through the Centers for Disease Control and Prevention’s (CDC’s) Active Bacterial Core Surveillance system (ABCs). Since 2013, however, increases in serotype 4 IPD cases were noted (unpublished data). We evaluated trends in serotype 4 IPD incidence, geographic distribution of serotype 4 strains, and genetic characteristics and relatedness of these strains identified in the post-PCV13 era.
METHODS
IPD Case Identification
ABCs, an active population- and laboratory-based system, defined cases of IPD as isolation of Streptococcus pneumoniae from a normally sterile site in a surveillance area resident. The surveillance areas covered entire states or select counties in 10 states, representing approximately 34 million individuals, in 2018 [3]. Case medical charts were reviewed to attain demographic, including residence type, and clinical information. During 2010 to 2015, ABCs categorized patients as persons experiencing homelessness (PEH) if they were documented in the medical record as homeless or residing in a shelter. During 2016–2018, ABCs’ definition of homeless was expanded to include patients who resided in a mission, medical respite, or church community center at the time of positive culture.
Isolates were assigned serotypes at CDC’s Streptococcus Laboratory using Quellung (1998–2015) and whole-genome sequencing (WGS; 2015–2018).
ABCs Data
ABCs data were determined by CDC human research protection procedures to be nonresearch, public health surveillance activities, and institutional review board review was not required. Informed consent was not required.
IPD Incidence
We calculated annual IPD incidence rates (cases per 100 000 population) using ABCs serotype 4 IPD cases as numerator and US Census Bureau population estimates as denominators [4]. For this analysis, we included serotype 4 cases identified from 1 January 2010 through 31 December 2018 in 10 continuously participating ABCs sites (selected counties in California, Colorado, Georgia, Maryland, New York, Oregon, Tennessee, and the entire states of Connecticut, Minnesota, and New Mexico). The ABCs populations under surveillance ranged from 29 757 552 (2010) to 34 460 237 (2018) persons. The surveillance areas were consistent during 2010–2018 for all sites except for California [3].
We estimated annual incidence of IPD caused by serotype 4 among PEH by restricting the analysis to ABCs cases reported among PEH. For the denominators, we used US Department of Housing and Urban Development (HUD) point-in-time count (PIT) data to enumerate the population of people experiencing homelessness in the 3 corresponding ABCs geographic areas for the years 2010–2018 [4]. The PIT is a yearly count of people experiencing sheltered or unsheltered homelessness conducted on a single night in January, as required by HUD for communities receiving federal funds from the McKinney-Vento Homeless Assistance Grants program. In these counts, a person is considered homeless if they sleep in an emergency shelter or transitional housing, or in a place not meant for human habitation such as on the street, in abandoned buildings, or in vehicles [5].
We compared the proportion of underlying risk factors (substance abuse, smoking, and one of more chronic underlying conditions) among PEH and non-PEH cases. Substance abuse was defined as past or current alcohol abuse, past or current history of intravenous drug use, or other drug use as reported in medical chart. Chronic medical conditions included chronic heart, lung, or liver disease, diabetes mellitus, congenital or acquired asplenia, sickle cell disease, chronic renal failure, congenital and acquired immunodeficiencies, solid organ malignancy, infection with HIV, Hodgkin disease, leukemia, lymphoma, multiple myeloma, nephrotic syndrome, or solid organ transplant.
WGS and WGS-Based Predictions
WGS analysis included serotype 4 IPD case isolates recovered during 2015–2018. Library construction, Illumina sequencing, and construction of draft genome assemblies was performed as described [6–8]. WGS accessions and relevant genomic features for serotype 4 isolates recovered during 2018 are provided (Supplementary Table 1) and this information for those recovered during 2015–2017 is also available ([9, 10]). Capsular serotypes, antimicrobial genotypes/phenotypes, 7 locus multilocus sequence type (ST), and pili (presence or absence) for year 2015–2018 isolates were deduced through our bioinformatics pipeline [6–10] for which periodic updates are available [11].
Phylogeny
Maximum likelihood trees were generated for the core genome shared between isolates, employing kSNP3.0 [12] with a kmer size of 19, and the MEGA7 program [13].
Statistical Analysis
Fisher exact test was used to compare serotype 4 incidence rates in 2010 with those in 2018. Differences were considered statistically significant at P < .05 (2-sided P values). Fisher exact tests were also used to compare proportions of serotype 4 IPD patients with underlying risk factors, comparing patients experiencing homelessness to those not experiencing homelessness.
RESULTS
Changes in Serotype 4 IPD, 2010–2018
Serotype 4 IPD incidence for all ABCs areas remained low during 2010–2018, but increases were noted from 0.1 cases/100 000 in 2010 (36 cases, all from individuals ≥17 years of age) to 0.3 cases/100 000 in 2018 (105 cases, all from individuals ≥18 years). Among children <18 years of age, 7 cases were reported during this time period (only 1 case during 2016–2018). Among adults ≥18 years of age, incidence increased from 0.2 in 2010 (35 cases reported) to 0.4 cases/100 000 in 2018 (104 cases reported).
From 400 serotype 4 IPD cases reported during 2010–2018, 328 (82%) were among those reported in 3 ABCs sites, California (CA), Colorado (CO), and New Mexico (NM) (Table 1). Serotype 4 IPD incidence increased in these sites, while no changes or reductions were observed in the remaining sites (Figure 1). All of the increases in type 4 IPD incidence in these 3 sites were among adults ≥18 years old (Figure 2A). Between 2010 and 2018, incidence of type 4 IPD among adults ≥18 years old increased from 0.7 to 1.2 cases/100 000 in CA (P = .32), 0.1 to 2.1 cases/100 000 in CO (P < .01), and 0.1 to 1.2 cases/100 000 in NM (P = .01).
Table 1.
Distribution of ABCs Serotype 4 Isolates Recovered Overall and From Persons Experiencing Homelessness by Reporting Year, 2010–2018
| No. of Serotype 4 Isolates by Year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
| All ABCs | 36 | 27 | 20 | 34 | 37 | 36 | 41 | 64 | 105 |
| Among PEH | 2 | 6 | 5 | 5 | 12 | 18 | 11 | 24 | 36 |
| CA, CO, NM | 8 | 14 | 15 | 27 | 30 | 30 | 37 | 64 | 103 |
| Among PEH in CA, CO, NM | 1 | 3 | 4 | 5 | 12 | 18 | 10 | 24 | 30 |
Abbreviations: ABC, Active Bacterial Core surveillance; CA, California; CO, Colorado; NM, New Mexico; PEH, persons experiencing homelessness.
Figure 1.
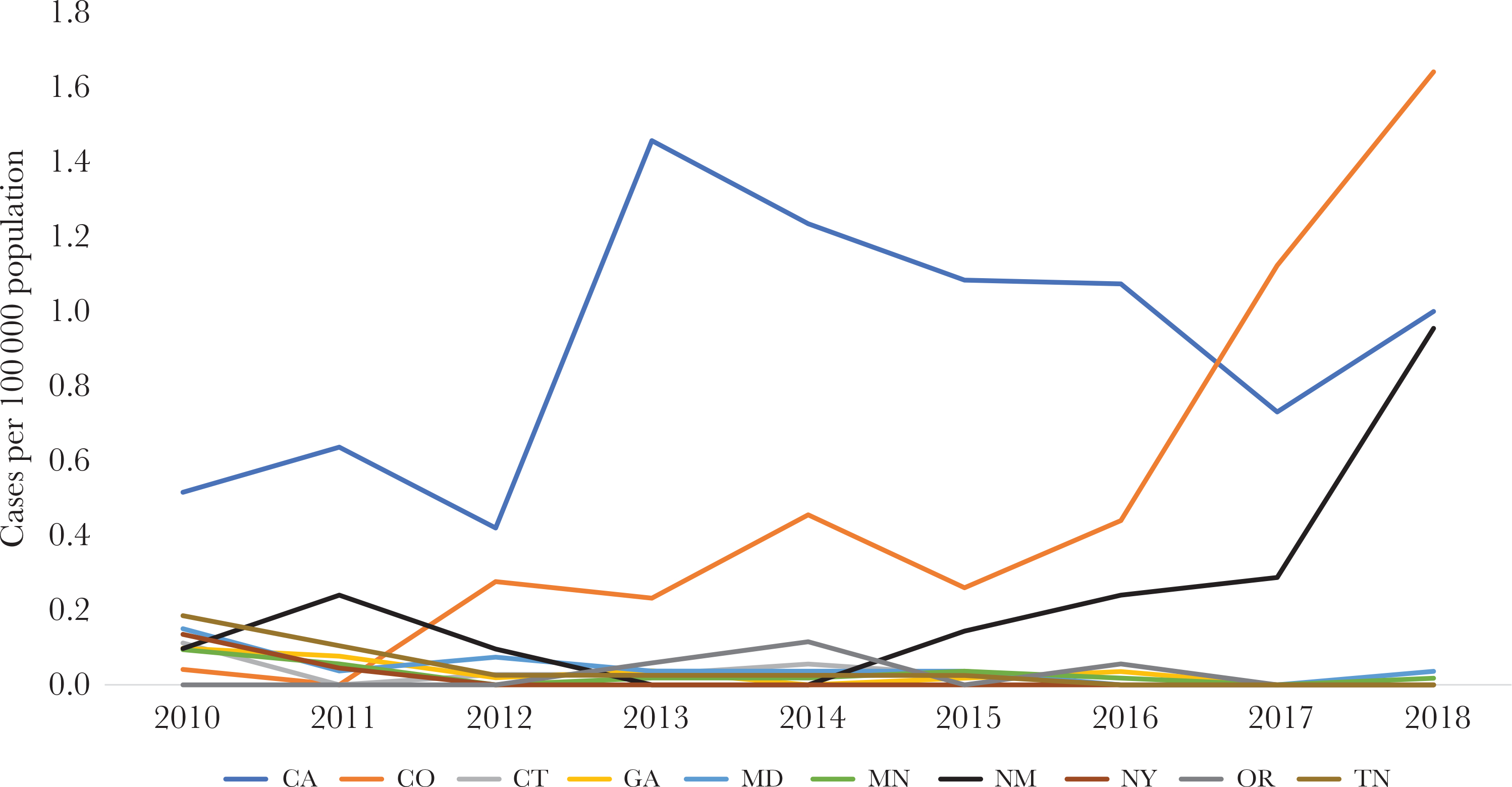
Incidence of serotype 4 invasive pneumococcal disease by Active Bacterial Core surveillance state, 2010–2018. Abbreviations: CA, California; CO, Colorado; CT, Connecticut; GA, Georgia; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; OR, Oregon; TN, Tennessee.
Figure 2.
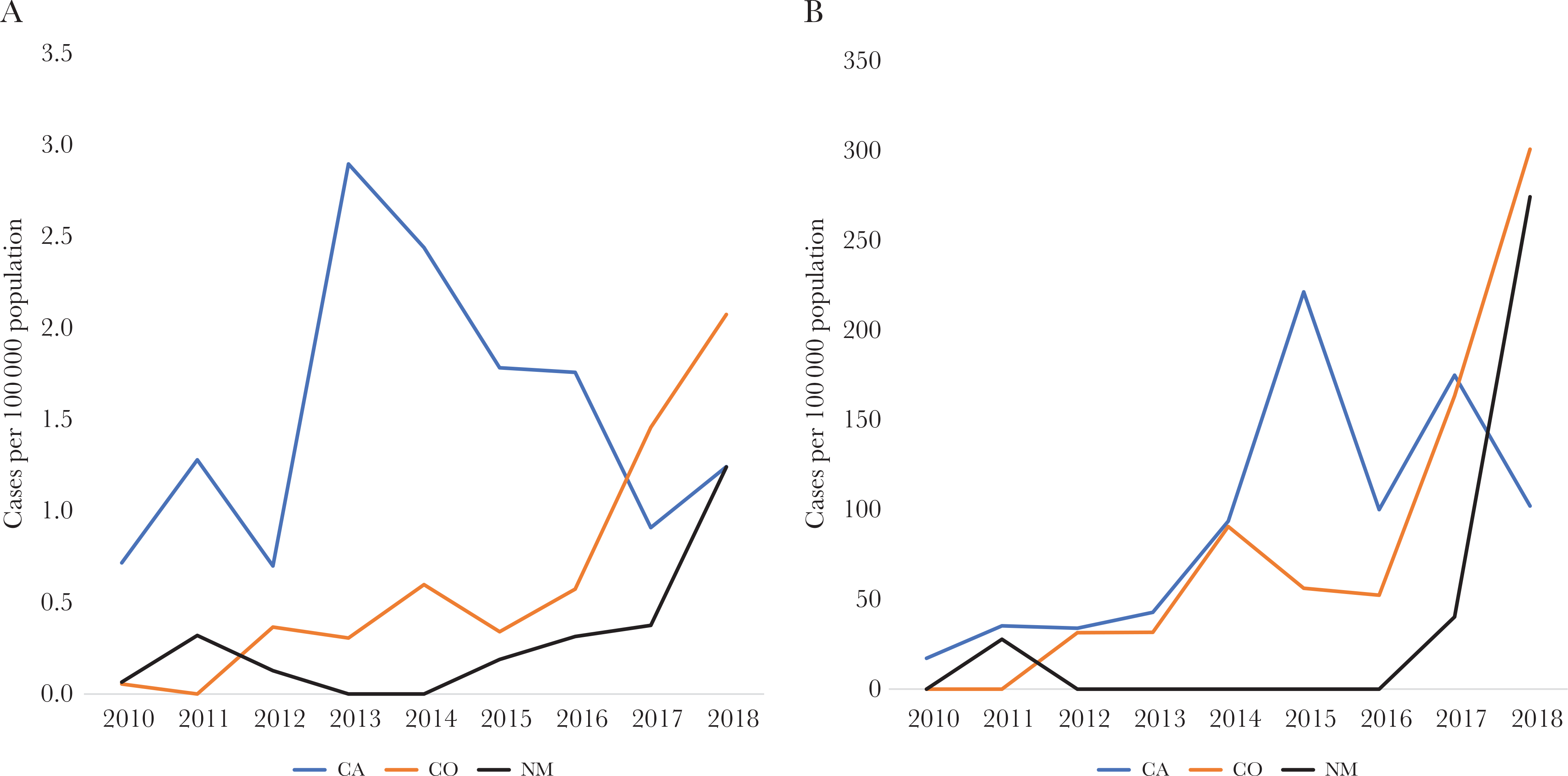
Incidence of serotype 4 invasive pneumococcal disease among adults 18 years or older in the general population (A) and persons experiencing homelessness (B) in 3 Active Bacterial Core surveillance sites, 2010–2018. Abbreviations: CA, California; CO, Colorado; NM, New Mexico.
Association of Serotype 4 in CO, CA, and NM With Persons Experiencing Homelessness
Among the 328 serotype 4 IPD cases identified at the 3 sites (CA, CO, and NM), 317 (97%) were of known residence status, 314 were among adults ≥18 years of age, 113 (36%) of which were among PEH (Table 1). Seventy-eight (69%) of PEH vs 116 (58%) of non-PEH with type 4 IPD had history of substance abuse (P = .05); 63 (56%) of PEH vs 129 (64%) of non-PEH had history of smoking (P = .15), and 59 (52%) of PEH vs 107 (53%) of non-PEH had 1 or more underlying chronic medical conditions reported (P = .9). The most prevalent underlying condition among PEH with serotype 4 IPD was HIV (29%), followed by chronic lung disease (17%) and diabetes mellitus (10%). Among non-PEH with serotype 4 IPD, the most prevalent underlying conditions were chronic lung disease (24%), HIV (14%), and diabetes (11%).
We estimated incidence of serotype 4 IPD among PEH and evaluated changes between 2010 and 2018. Among PEH from the 3 states aged 18 years or older, incidence of serotype 4 IPD increased from 17 cases per 100 000 PEH in 2010 to 221 per 100 000 PEH in 2015 and 102 per 100 000 PEH in 2018. (Figure 2B). From 2010 to 2018, incidence increased from 0 to 301 per 100 000 PEH in CO and 0 to 274 per 100 000 PEH in NM. In 2018, incidence was 100–300 times higher among PEH vs non-PEH in these states.
Serotype 4 Strain Feature Summary
The 246 isolates recovered during 2015–2018 (242 from individuals ≥18 years of age, 4 from ages 7–16 years) were primarily of 3 distinct multilocus STs (ST10172, ST244, and ST695) (Table 2), and 11 isolates were single-locus variants of one of the major STs. Four isolates were of the fourth distinct ST, ST12920, ST205, and ST247 occurred in individual isolates. All isolates were predicted by penicillin binding protein sequences to be susceptible to beta-lactam antibiotics and the majority (204/246, 82.9%) of isolates were cotrimoxazole-resistant due to mutations within folP and/or folA. Three ST695 outliers recovered in nonwestern states were erythromycin resistant (mef-positive). Two ST12920 isolates were resistant to erythromycin, clindamycin, chloramphenicol, and tetracycline (positive for ermB, cat, and tetM). Two ST10172 isolates were resistant to erythromycin and clindamycin only (ermB-positive). All serotype 4 isolates other than those identical or related to ST10172 and ST12920 were positive for the major pilus-1 backbone protein determinant rrgA.
Table 2.
Distribution of ABCs Serotype 4 Isolates Recovered Overall and From Persons Experiencing Homelessness by Lineage, 2015–2018
| Serotype 4 Lineage | No. Isolated by Year (PEH) |
||||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | total | |
| ST10172 | |||||
| CA | 0 | 0 | 0 | 1 (0) | 1 (0) |
| CO | 7 (3) | 12 (3) | 31 (9) | 44 (15) | 94 (30) |
| NM | 3 (0) | 5 (0) | 6 (1) | 21 (7) | 35 (8) |
| Other states | 1 (0), MD | 1 (0), OR | 0 | 0 | 2, MD, OR |
| ST244 | |||||
| CA | 19 (14) | 16 (7) | 15 (12) | 22 (10) | 72 (43) |
| CO | 0 | 0 | 0 | 2 (1) | 2 (1) |
| NM | 0 | 0 | 0 | 0 | 0 |
| Other states | 0 | 0 | 0 | 0 | 0 |
| ST695 | |||||
| CA | 0 | 4 (0) | 12 (2) | 9 (3) | 25 (5) |
| CO | 0 | 0 | 0 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 |
| Other states | 2 (0), MN | 2 (1), GA | 0 | 1 (0), MD | 5 (1), MN, GA, MD |
| ST205 | |||||
| CA | 0 | 0 | 0 | 1 (0) | 1 (0) |
| CO | 0 | 0 | 0 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 |
| Other states | 2 (0), GA, TN | 1 (0), MN | 0 | 1 (0), MN | 4 (0), MN, GA, TN |
| ST2920 | |||||
| CA | 0 | 0 | 0 | 4 (0) | 4 (0) |
| CO | 0 | 0 | 0 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 |
| Other states | 0 | 0 | 0 | 0 | 0 |
| ST247 | |||||
| CA | 0 | 0 | 0 | 0 | 0 |
| CO | 0 | 0 | 0 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 |
| Other states | 1 (0), CT | 0 | 0 | 0 | 1 (0), CT |
| Totals | 35 (17) | 41 (11) | 64 (24) | 106 (36) | 246 (88) |
Abbreviations: CA, California; CO, Colorado; CT, Connecticut; GA, Georgia; MD, Maryland; MN, Minnesota; NM, New Mexico; OR, Oregon; PEH, persons experiencing homelessness; TN, Tennessee.
Distribution of Serotype 4 Clonal Complexes in ABCs During 2015–2018
There were 6 distinct clonal lineages, each with a primary multilocus ST (Table 2). These 6 STs differed from each other by 3 or more loci. Three of these lineages (ST10172, ST244, and ST695) accounted for 236 (95.9%) of the total 246 serotype 4 isolates identified in 2015–2018. All but 7 of the isolates from these 3 major lineages were recovered within 3 states (CO, CA, and NM). All but 3 serotype 4 isolates recovered in MN and MD were from individuals ≥18 years of age. In the pre-PCV7 and early post-PCV7 periods (1999–2002), only multilocus ST genotypes identical or highly related to ST695, ST244, and ST205 were detected among serotype 4 ABCs isolates [14, 15]. STs 695 and 244 are 3-locus variants, with ST205 differing in 4 of 7 multilocus ST loci with ST244.
CO and NM Lineage ST10172
Multilocus sequence type ST10172 (ST10172) is a double-locus variant of ST437 and of ST1157 [16], both of which are within the major penicillin-susceptible serotype 23A clonal complex CC439 [9]. During 2015–2018, the ST10172 lineage accounted for 132 (53.9%) of the total type 4 isolates, 38 (28.8%) of which were isolated from PEH. With the exception of 3 isolates (from CA, MD, and OR), all ST10172 lineage isolates were recovered from CO (94/132, 71.2%) or NM (34, 26.0%) (Table 2).
ST10172 was first discovered from an infant in ABCs during 2013 [6], and was postulated to be a recently emerged serotype switch variant, because there was no previous history of this clonal complex within serotype 4 [16]. During the period 2012–2014, prior to implementation of WGS-based surveillance for ABCs, it is likely that type 4/ST10172 emerged in small numbers in CO prior to its observed emergence during 2015–2018 (Table 2). Serotype 4/ST10172 strains started to appear in small numbers in NM during 2015 and have shown more rapid emergence starting in 2017 (Table 2). These type 4/ST10172 isolates recovered in NM are apparently highly temporally related to those causing IPD in CO during 2015–2018 on the basis of small single-nucleotide polymorphism (SNP) differences (Figure 3A).
Figure 3.
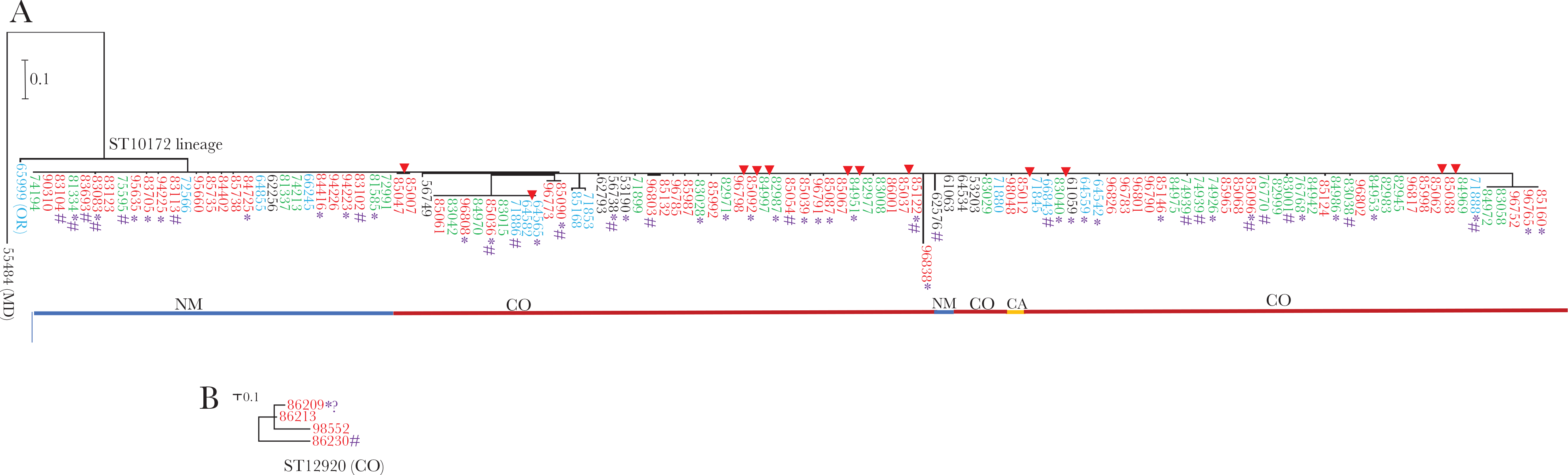
Phylogenetic analysis by maximum likelihood method of ST10172 lineage (A) and ST12920 (B) core genomes primarily found in CO and NM. The year the isolate was recovered is indicated by font color (black, 2015; blue, 2016; green, 2017; red, 2018). As indicated below the ST10172 lineage tree, the majority of isolates were recovered in CO and NM, with single isolates of ST10172 lineage recovered in MD (left outlier) and CA. The 4 ST12920 isolates were recovered in CO. Homeless individuals are indicated by an asterisk and injection drug abuse is indicated by #. Eight sets of 2–4 genetically identical (0 SNPs) isolates are indicated with red arrows. The evolutionary history was based on the general time reversible model [13]. The trees with the highest log likelihoods are shown. The trees are drawn to scale, with branch lengths measured in the number of substitutions per site. The ST10172 tree includes 123 genome assemblies with a total of 2560 positions in the final dataset and the ST12920 involved 4 assemblies with 66 positions. Abbreviations: CA, California; CO, Colorado; MD, Maryland; NM, New Mexico; SNP, single-nucleotide polymorphism.
Of the 131 ST10172 isolates, 123 yielded WGS assemblies of sufficient quality for phylogenic analysis (Figure 3A). High relatedness was shared between isolates within the major phylogenetic cluster (depicted by the long straight line). Of the 123 isolates, 113 (91.2%) differed by 10 or fewer SNPs from at least 1 other isolate depicted, including 99 (80.5%) with 5 or fewer SNPs. There were 8 sets of 2–4 isolates that were genetically indistinguishable (0 SNPs). The arithmetic mean of all individual pairwise SNP differences between the 123 isolates was 57 SNPs; however, this includes 1 outlier as well as 2 small clusters that are relatively distant and each composed of 5–9 highly related individual isolates (Figure 3).
CO Lineage ST12920
During 2018 another distinct clonal complex, ST12920, appeared among 4 serotype 4 isolates recovered in CO (Table 2 and Figure 3B), with pair-wise differences ranging from 10–54 SNPs. Information regarding this genetic lineage is currently restricted to invasive serotype 4 isolates recovered in Japan and China [16].
CA Lineages ST244 and ST695
Of the 109 isolates within these 2 lineages, 102 (93.6%) were confined to CA (Table 2, Figure 4). Although this genomic information pertaining to these isolates is lacking for years 2010–2014, it is possible that IPD in CA due to serotype 4 of lineages ST244 and ST695 started to increase soon after PCV13 implementation.
Figure 4.
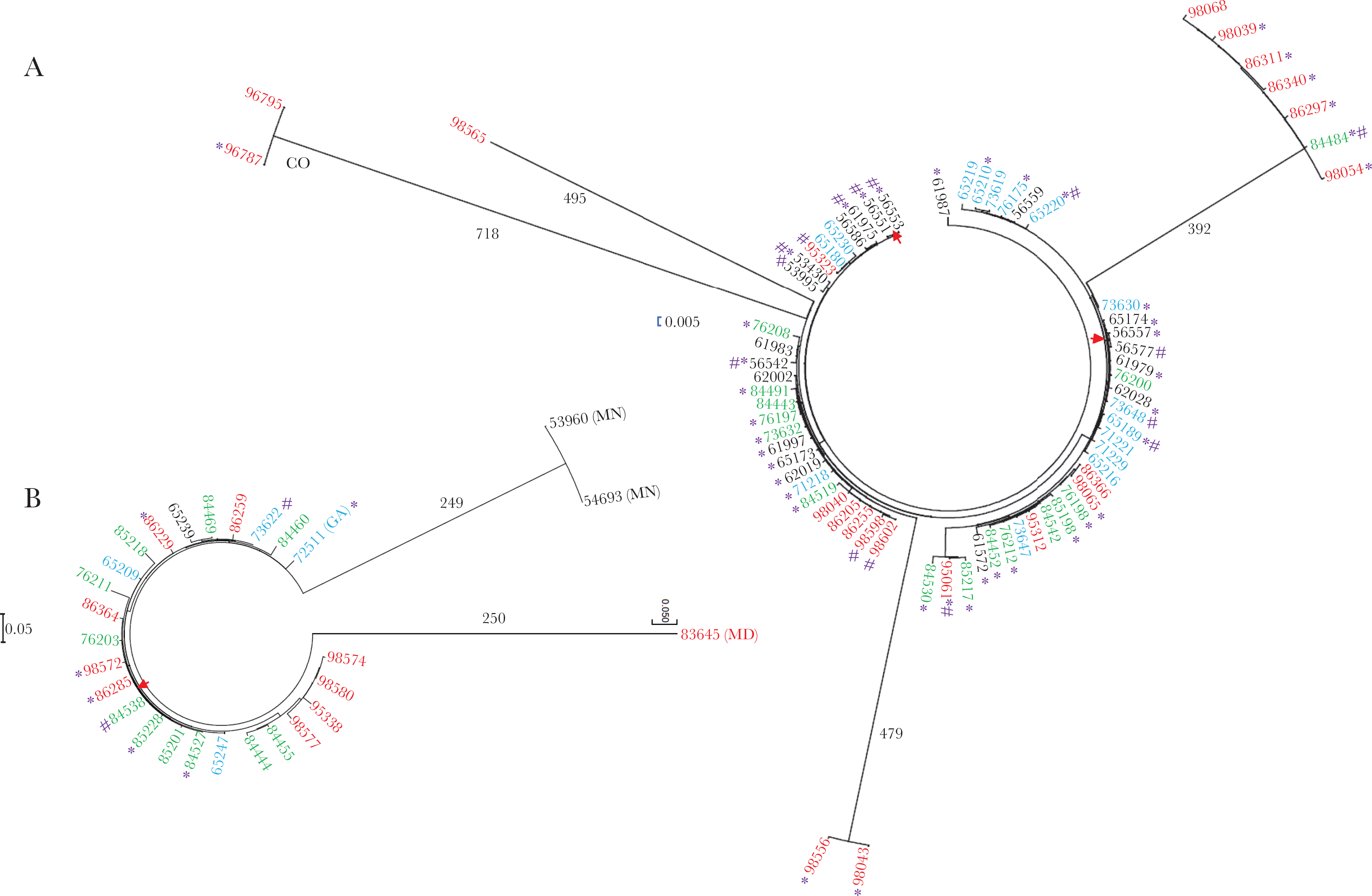
Phylogenetic analysis by maximum likelihood method of ST244 lineage (A) and ST695 (B) core genomes. All isolates except for 2 ST244 lineage isolates (outliers from CO) and 4 ST695 lineage isolates (outliers from MN and MD, and a single GA isolate) were recovered in CA. The year the isolate was recovered is indicated by font color (black, 2015; blue, 2016; green, 2017; red, 2018). Homeless individuals are indicated by an asterisk and injection drug abuse is indicated by #. Trees were constructed to scale. Red arrows are situated between genetically indistinguishable isolates. A, included 99 assemblies and 954 positions, while (B) involved 28 assemblies and 647 positions. SNP values are provided for 4 long branches (A) and 2 long branches (B). Abbreviations: CA, California; CO, Colorado; GA, Georgia; MD, Maryland; MN, Minnesota; SNP, single-nucleotide polymorphism.
Following a plateau period (2015–2017), there has been a modest increase of the long-standing serotype 4/ST244 lineage that has been confined to CA (Table 2). The majority (59.5%, 44/74) of the serotype 4/ST244 isolates were recovered from PEH. Of the 74 ST244 lineage isolates, 71 yielded high-quality assemblies for phylogenetic analysis (Figure 4A). Of these 71 isolates, 62 (87.3%) differed by 10 or fewer SNPs with at least 1 other isolate depicted, 32 (45.1%) of which differed by 5 or fewer SNPs from other isolates shown. There were 2 pairs of isolates that were genetically indistinguishable; these 4 isolates were recovered from PEH and/or with history of drug abuse. The ST244 phylogram appears to be indicative of multiple unrelated disease clusters, as evidenced by 3 highly related small clusters that diverged from the majority of isolates by >392 SNPs.
ST695, formerly the most common genotype within pre-PCV7 serotype 4 IPD isolates [14] and the recipient genotype of an important serotype 19A capsular switch variant detected shortly after PCV7 implementation [15, 17], was also largely restricted to CA during 2015–2018. Of the 28 serotype 4/ST695 genomes analyzed, 22/28 (78.6%) differed by 10 or fewer SNPs from at least 1 other isolate within the phylogram (Figure 4B), and 16 (57.1%) differed by 5 or fewer SNPs from other isolates. These included 2 isolates that were genetically identical (1 from a PEH and 1 from a person with a history of drug abuse).
DISCUSSION
During 2010–2018, we documented increases in IPD incidence caused by vaccine serotype 4 in 3 geographic areas in the United States. While these increases were observed in the general population of adults, the most dramatic changes in serotype 4 IPD were observed among adult populations experiencing homelessness. In 2018, incidence of serotype 4 IPD among PEH was up to 300 times higher than in the general population within the same geographic areas. Even so, this is likely to be an underestimate because homelessness in ABCs is obtained from medical record review and is unlikely to be accurately reported for all IPD cases. The regional increase in serotype 4 IPD within ABCs western states is reminiscent of the emergence of emm59 that was observed in NM during the year 2015 in ABCs invasive group A streptococcal (GAS) disease surveillance [18]. As with the current emergence of serotype 4 pneumococci, the emergence of emm59 and of other relatively uncommon GAS strains has been highly associated with homelessness and injection drug use within western ABCs sites (ABCs unpublished data). There are many potential consequences of homelessness, which include inadequate hygiene and over-crowded living conditions within shelters that predispose for airborne infections. Homeless individuals are also much more likely than non-PEH to report substance abuse and have underlying chronic medical conditions present, leading to increased risk for infections. PEH are at increased risk for infections due to limited access to healthcare and preventive care, such as vaccinations [19, 20].
The unusual geographically localized increases of serotype 4 IPD within 3 of the 10 ABCs sites appears related to a particularly vulnerable subset of individuals within these surveillance sites, rather than being caused by any specific virulence properties inherent to these 3 major unrelated serotype 4 lineages (other than the serotype 4 capsule itself). The CO, NM, and CA ABCs sites have each experienced dramatic increases of individuals experiencing homelessness [5]. In CA, serotype 4 cases were from the Bay Area counties of San Francisco (67 isolates), Alameda (25 isolates), and Contra Costa (11 isolates), each of which have experienced dramatic increases of individuals experiencing homelessness [21]. Only San Francisco County conducted surveillance among adults during the entire 2010–2018 period. San Francisco’s 2019 total homeless population is currently reported as the highest in the Bay area. Contra Costa and Alameda are neighboring counties that have reported an increase of their homeless population of 43% between 2017 and 2019. Of CO isolates, the largest fraction (51 isolates) were from Denver county, which has undergone a 22% increase of its homeless population during 2014–2019 [22]. NM reportedly has the highest national rate of chronic homelessness in the United States, with an increase of 2.8% since 2017 [23]. As in our study, serotype 4 caused a much higher proportion of disease among PEH compared to non-PEH in Canadian studies undertaken during the post-PCV7 era [24, 25]. Another recent Canadian report described an outbreak of serotype 4 IPD in a homeless and unstably housed population [26]. In common with this report, our data calls attention to the need to improve pneumococcal disease prevention among this disadvantaged population.
There is a limitation to our analysis of serotype 4 IPD among PEH. Although it is likely that the 2016 expansion in ABCs definition of homelessness may have improved ascertainment of PEH status, misclassification of PEH status is still likely. Some of the observed increases in serotype 4 incidence among non-PEH could be attributed to misclassification of PEH cases as non-PEH. In addition, we were not able to find differences in the prevalence of risk factors, such as injection drug use, smoking, and prevalence of underlying medical conditions between serotype 4 IPD cases among PEH vs non-PEH. Because we were not able to assess the distributions of these characteristics in the underlying population, our ability to assess the difference in risk factors for disease between PEH and the general population was limited.
Serotype 4 polysaccharide is included in PCV7 and PCV13, and efficacy of both vaccines in preventing pneumococcal disease and nasopharyngeal carriage caused by this serotype has been demonstrated [27, 28]. Pneumococcal infections caused by serotype 4 have been dramatically reduced since 2000, and nasopharyngeal carriage surveys conducted in post-PCV7 and post-PCV13 years show that this serotype is rarely carried among children in the United States [29]. While the benefits of PCV in reduction of disease caused by vaccine serotypes, including serotype 4, remain in the general population, recent increases in serotype 4 IPD in select geographic areas among PEH suggest that a low-level transmission of vaccine types in the community is still ongoing after 2 decades of PCV use in the United States. This vulnerable group of individuals is evidently at increased risk for exposure to and infections caused by these strains. Pneumococcal 23-valent polysaccharide vaccine (PPSV23) and PCV13 are recommended, alone or in series, for a select group of US adults, depending on the age and presence of underlying chronic medical conditions. Pneumococcal vaccines have been recommended in a setting of outbreaks among PEH [30], but currently pneumococcal vaccines are not routinely recommended for PEH. Homelessness is associated with substantial health inequalities, including poor access to healthcare, resulting in higher morbidity due to preventable conditions [20, 24]. Close monitoring of pneumococcal serotypes causing disease among PEH is needed to understand the direct and indirect effects of PCV and determine if future vaccine policy discussions should include homelessness as an indication for pneumococcal vaccines.
Supplementary Material
Acknowledgments.
We are grateful to A. Reingold, S. Brooks, H. Randel, L. Miller, B. White, D. Aragon, M. Barnes, J. Sadlowski, S. Petit, M. Cartter, C. Marquez, M. Wilson, M. Farley, S. Thomas, A. Tunali, W. Baughman, L. Harrison, J. Benton, T. Carter, R. Hollick, K. Holmes, A. Riner, R. Lynfield, P. Vagnone-Snippes, A. Glennen, C. Holtzman, R. Danila, K. MacInnes, K. Scherzinger, K. Angeles, J. Bareta, L. Butler, S. Khanlian, R. Mansmann, M. Nichols, N. Bennett, S. Zansky, S. Currenti, S. McGuire, A. Thomas, M. Schmidt, J. Thompson, T. Poissant, W. Schaffner, B. Barnes, K. Leib, K. Dyer, L. McKnight, O. Almendares, J. Hudson, H. Pham, G. Langley, and Melissa Arvay for their contributions to the establishment and maintenance of the ABCs system. This publication made use of the Streptococcus pneumoniae MLST website (https://pubmlst.org/spneumoniae/) sited at the University of Oxford.
Financial support.
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pilishvili T, Lexau C, Farley MM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 2.Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) https://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed 22 April 2020. [Google Scholar]
- 4.United States Census Bureau. Population and housing unit estimates. https://www.census.gov/programs-surveys/popest.html. Accessed 15 January 2020.
- 5.US Department of Housing and Urban Development. PIT and HIC data since 2007. https://www.hudexchange.info/resource/3031/pit-and-hic-data-since-2007/. Accessed 15 January 2020.
- 6.Metcalf BJ, Gertz RE Jr, Gladstone RA, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect 2016; 22:60.e9–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metcalf BJ, Chochua S, Gertz RE Jr, et al. Using whole genome sequencing to identify resistance determinants and predict antimicrobial resistance phenotypes for year 2015 invasive pneumococcal disease isolates recovered in the United States. Clin Microbiol Infect 2016; 22:1002.e1–e8. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Metcalf BJ, Chochua S, et al. ; Active Bacterial Core Surveillance Team. Validation of β-lactam minimum inhibitory concentration predictions for pneumococcal isolates with newly encountered penicillin binding protein (PBP) sequences. BMC Genomics 2017; 18:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beall B, Chochua S, Gertz RE Jr, et al. A population-based descriptive atlas of invasive pneumococcal strains recovered within the U.S. During 2015–2016. Front Microbiol 2018; 9:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect 2020; 26:512.e1–e10. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf BJ. Database update. https://github.com/BenJamesMetcalf/Spn_Scripts_Reference. Accessed 19 August 2020.
- 12.Gardner SN, Slezak T, Hall BG. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 2015; 31:2877–8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertz RE Jr, McEllistrem MC, Boxrud DJ, et al. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J Clin Microbiol 2003; 41:4194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B; Active Bacterial Core Surveillance Team. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis 2005; 192:1988–95. [DOI] [PubMed] [Google Scholar]
- 16.PubMLST. Streptococcus pneumoniae MLST databases. https://pubmlst.org/spneumoniae/. Accessed 19 August 2020.
- 17.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 2007; 3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chochua S, Metcalf BJ, Li Z, et al. Population and whole genome sequence based characterization of invasive group a streptococci recovered in the United States during 2015. mBio 2017; 8:e01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambatese M, Marder D, Begier E, et al. Programmatic impact of 5 years of mortality surveillance of New York City homeless populations. Am J Public Health 2013; 103(suppl 2):S193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baggett TP, O’Connell JJ, Singer DE, Rigotti NA. The unmet health care needs of homeless adults: a national study. Am J Public Health 2010; 100:1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ABC News. History of how many people are homeless in the Bay Area. https://abc7news.com/society/homeless-population-history-in-bay-area/5260657/. Accessed 19 August 2020.
- 22.Denver Post. Efforts to combat homelessness in Denver’s suburbs as problem persists. https://www.denverpost.com/2019/08/27/homelessness-suburbs-jefferson-arapahoe-adams/. Accessed 19 August 2020.
- 23.US News. Homelessness up in New Mexico, state No. 1 for severe cases. https://www.usnews.com/news/best-states/new-mexico/articles/2018-12-18/new-mexico-sees-uptick-in-homelessness. Accessed 19 August 2020.
- 24.Plevneshi A, Svoboda T, Armstrong I, et al. ; Toronto Invasive Bacterial Diseases Network. Population-based surveillance for invasive pneumococcal disease in homeless adults in Toronto. PLoS One 2009; 4:e7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemay JA, Ricketson LJ, Zwicker L, Kellner JD. Homelessness in adults with invasive pneumococcal disease (IPD) in Calgary, Canada. Open Forum Infect Dis 2019; 6:ofz362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKee G, Choi A, Madill C, Marriott J, Kibsey P, Hoyano D. Outbreak of invasive Streptococcus pneumoniae among an inner-city population in Victoria, British Columbia, 2016–2017. Can Commun Dis Rep 2018; 44:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19:187–95. [DOI] [PubMed] [Google Scholar]
- 28.Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis 2001; 1:85–91. [DOI] [PubMed] [Google Scholar]
- 29.Desai AP, Sharma D, Crispell EK, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J 2015; 34:1168–74. [DOI] [PubMed] [Google Scholar]
- 30.Schillberg E, Isaac M, Deng X, et al. Outbreak of invasive Streptococcus pneumoniae serotype 12F among a marginalized inner-city population in Winnipeg, Canada, 2009–2011. Clin Infect Dis 2014; 59:651–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


