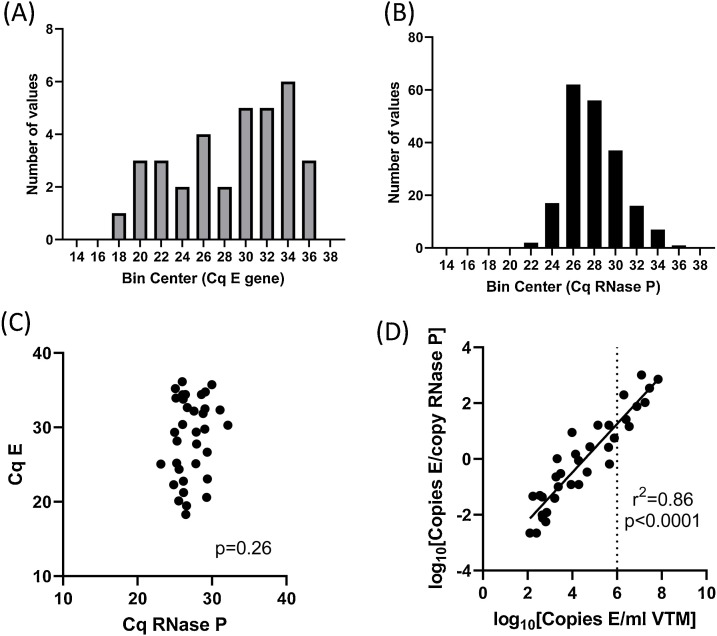
Fig. 5.
Distribution of E and RNase P Cq in clinical samples.
Clinical samples (n = 204) were assayed using the E primers and FAM-labelled probe in combination with RNase P primers and HEX-labelled probe (Duplex). N = 34 samples had detectable SARS-CoV-2, 6 were classed as inadequate due to lack of amplification of RNAse P, and the remainder were classified as ‘SARS-CoV-2 not detected’. Histograms of Cq values for E gene in samples with detectable SARS-CoV-2 (A) and Cq values for RNase P in all adequate samples (B). (C) E versus RNAse P Cq values in all samples with detectable SARS-CoV-2. (D) Normalised copy number of E versus per-swab copy number. Dotted line indicates 1,000,000 copies/mL (Cq E gene = 24 in the duplex assay).