Figure 3.
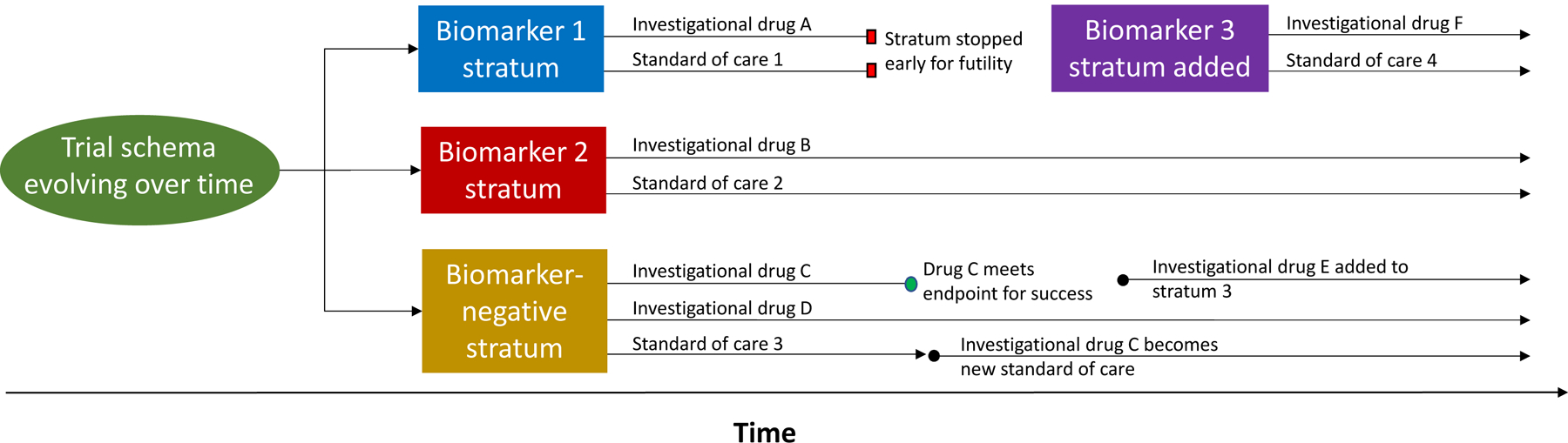
Example schema for an adaptive platform trial. The schema depicts evolution of a platform trial’s design over time. In this example, patients are screened and matched to a trial stratum based on the presence or absence of targetable biomarkers. Each stratum features one or more investigational therapies personalized to patient biomarker status compared to a standard of care. As evidence from the trial accrues, each stratum or arm within a stratum can be individually stopped early for success or futility, while remaining strata and arms may be left open for continued enrollment. New strata (e.g. Biomarker 3 stratum) and treatment arms (e.g. investigational drug E) can be added as the trial proceeds. If a stratum closes early, patients enrolled in that stratum can be enrolled in another (e.g. transition from Biomarker 1 stratum to the Biomarker-negative stratum). The overall trial does not necessarily feature a fixed stop date.
