Abstract
Microalbuminuria is a major independent risk factor for cardiovascular disease (CVD) events in persons with diabetes or hypertension, the general population, and persons with known CVD. Although microalbuminuria is a stronger risk factor in men, women with increased albuminuria levels are also at a higher risk of CVD. Microalbuminuria is an indicator of generalized endothelial injury, a hallmark of systemic atherosclerosis. Treatments that decrease albuminuria, particularly agents that inhibit the renin‐angiotensin system, reduce CVD risk in various populations, including those with and without diabetes or hypertension. Whether albuminuria should be a treatment target for CVD is not yet proven. Nevertheless, the measurement of albuminuria is clinically useful to identify high‐risk individuals who should receive intensive risk factor management based on current treatment guidelines.
The interest in microalbuminuria originated with the observation that it was a marker that could be used to identify the subset of persons with diabetes who had nephropathy and were at risk of a progressive loss of renal function. Approximately 30% of persons with type 1 diabetes and 40% of those with type 2 diabetes have microalbuminuria. However, in patients with type 2 diabetes, microalbuminuria is actually a stronger predictor of cardiovascular disease (CVD) than of renal outcomes (loss of glomerular filtration rate, end‐stage renal disease, or death). 1 The risk of CVD death has been reported to be increased by 100%–150%, depending on the level of increase in albuminuria. 1 Microalbuminuria is also common in hypertension, occurring in about 25% of patients. Like type 2 diabetes, albuminuria is a stronger predictor of CVD than of renal outcomes in hypertension, whereas in type 1 diabetes, renal outcomes predominates as a predictor of risk (Table I). In type 2 diabetes, microalbuminuria doubles the risk of major CVD events and mortality independent of conventional risk factors (Figure I). 2
Table I.
Prevalence and Predictive Value of Microalbuminuria in Diabetes and Hypertension
| Prevalence | Predictive Value for Outcome | |
|---|---|---|
| Mean (range) % | Cardiovascular | Renal |
| Hypertension 25 (5–40) | ++ | + |
| Type 2 DM 40 (10–80) | ++ | + |
| Type 1 DM 30 (5–50) | + | ++ |
| DM=diabetes mellitus | ||
Figure 1.
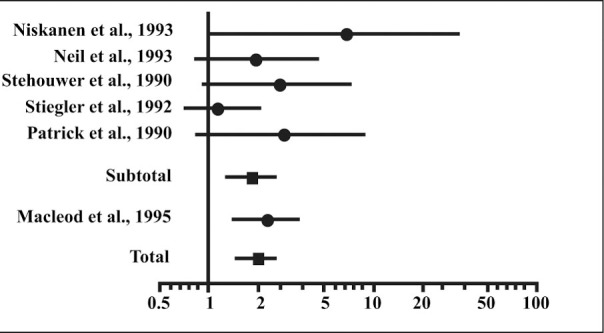
Association of microalbuminuria and cardiovascular morbidity and mortality in type 2 diabetes. Odds ratios of cardiovascular morbidity and mortality in patients with type 2 diabetes with microalbuminuria vs. normoalbuminuria. Reprinted with permission from Arch Intern Med. 1997;157:1413–1418. 2 Copyright 1997, American Medical Association. All Rights Reserved.
In hypertension, most ischemic heart disease events occur in the subset of patients who have microalbuminuria (Figure 2). 3 The prevalence of microalbuminuria is about 10% in the general population. As for high‐risk populations with diabetes and/or hypertension, increased levels of albuminuria also predict a marked increased risk of CVD events and deaths in the general population (Figure 3). 4 , 5
Figure 2.
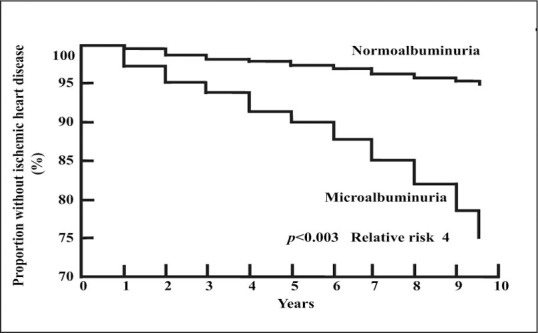
Survival without ischemic heart disease in hypertensive subjects by albuminuria status. Reprinted with permission from Hypertension. 2000;35:898–903. 3
Figure 3.
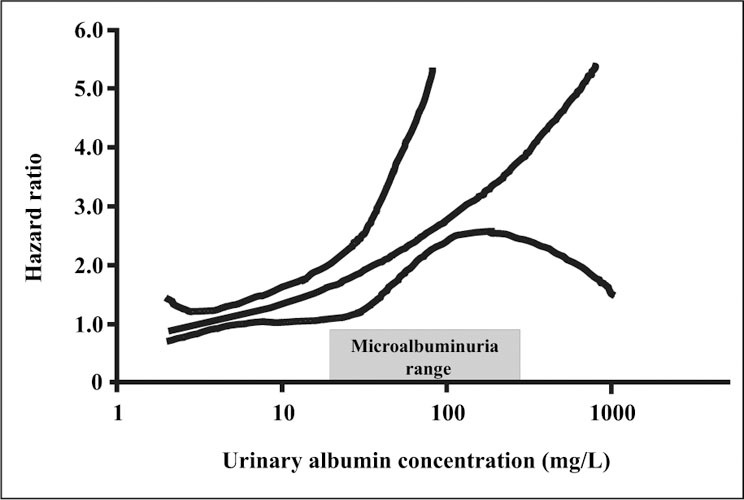
Risk of cardiovascular death by albuminuria level in the general population. Reprinted with permission from Circulation. 2002;106:1777–1782. 4
Microalbuminuria is associated with the metabolic syndrome, which includes the traits of abdominal obesity, glucose intolerance/hyperinsulinemia/insulin resistance, dyslipidemia (increased triglycerides/decreased high‐density lipoprotein cholesterol), and hypertension. With increasing numbers of metabolic syndrome traits, the risk of developing microalbuminuria increases steadily. 6 , 7 Accordingly, the prevalence of microalbuminuria increases progressively across the spectrum from normal glucose tolerance (10%) to impaired glucose tolerance (25%), to overt type 2 diabetes (40%). This increasing prevalence parallels increasing rates of CVD events, including myocardial infarction, left ventricular hypertrophy, stroke, peripheral vascular disease, and death across the metabolic syndrome spectrum. It is important to recognize that microalbuminuria is an independent contributor to these outcomes in the various populations studied.
The levels of albuminuria that predict CVD are lower than those that have traditionally been used to predict the risk of losing kidney function in diabetes (i.e., a urinary albumin‐to‐creatinine ratio (ACR) >30 mg/g). Levels of albuminuria increase progressively with the severity of coronary artery disease (Figure 4). 8 In a study of people undergoing elective coronary angiography, the average ACR was 10 mg/g in patients with normal arteries and 31 mg/g in individuals with severe (generally 3‐vessel) coronary artery disease. 8 ACR values were intermediate in patients with less severe degrees of coronary artery disease. Similarly, the risk of CVD events is increased at levels of albuminuria traditionally considered to be within the normal range. 9 Although still a matter of study and debate, ACR levels >15 mg/g can be considered indicative of increased CVD risk. Some studies have suggested that the ACR cut off for increased CVD risk may be even lower. For the first time, recent recommendations from the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure 10 recognize both microalbuminuria and a glomerular filtration rate of <60 mL/min as major CVD risk factors.
Figure 4.
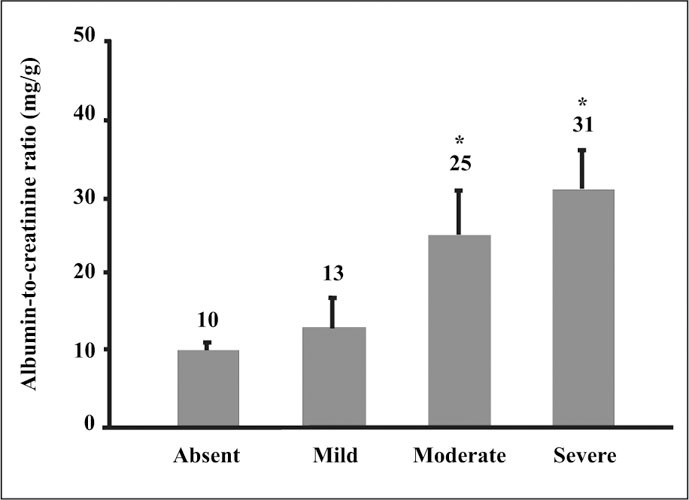
Urinary albumin and severity of coronary artery disease. *p=0.027 vs. Absent and Mild groups Reprinted from Am J Kidney Dis. 1999;34:918–925. Copyright 1999, with permission from the National Kidney Foundation. 8
The accumulating evidence indicates that microalbuminuria appears to be a stronger risk factor in men than in women; microalbuminuria occurs more frequently and is more closely associated with CVD and renal outcomes in men. Microalbuminuria is, however, also an important risk factor in women. In a large population‐based study, women with albuminuria levels in the highest compared with the lowest quintile had a greatly increased risk of CVD mortality over 20 years. 11
Another population‐based study found that the risk of microalbuminuria was increased about twofold with the administration of exogenous estrogen both in postmenopausal women (due to hormone replacement therapy) and premenopausal women (due to oral contraceptive use). 12 Risk was also related to dose and duration of estrogen use in that study. Such information adds to the concerns about the administration of estrogen, particularly in individuals who are already at a high CVD risk.
MICROALBUMINURIA AS A WINDOW TO THE CIRCULATION: MECHANISTIC CONSIDERATIONS
Although increases in albuminuria have long been known to reflect progressive kidney damage, this marker also appears to be an excellent indicator of the status of circulation at large. Increased amounts of albumin are lost in the urine when endothelial cells are damaged in the glomerulus. This damage may be the result of multiple pathways to injury, including hyperglycemia, hypertension, and dyslipidemia. The glomerulus is a unique capillary structure that has an arteriole on both its afferent and efferent ends (Figure 5). By contrast, all other capillary circulations have an arteriole in the afferent position with a venule on the efferent side. The unique structure of the glomerulus results in a higher blood pressure than in typical capillaries, as well as other characteristics with similarities to vessels of the arterial circulation. At a cellular level, damage to the glomerulus and to arteries can be viewed as parallel biological processes (Table II). Regardless of the inciting cause of injury, the responses of the various cells involved in both athero‐ and glomerulosclerosis are largely shared. After the initial endothelial injury, a cascade of pro‐sclerotic events ensues. In particular, the smooth muscle cells in atherosclerosis behave much like their corresponding contractile cell in the glomerulus, the mesangial cell. Both of these cell types proliferate, migrate from the subendothelial location to the vessel lumen, change phenotype, and secrete growth factors and matrix proteins.
Figure 5.
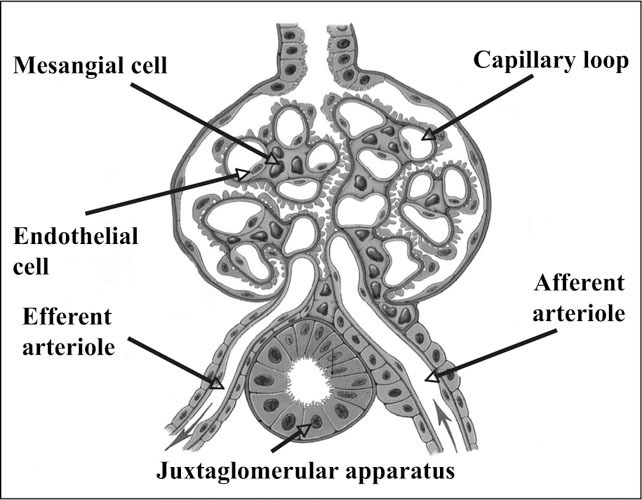
Glomerular structure Reprinted from Tuttle KR, Stein JH. Diabetic nephropathy. Current Concepts. Kalamazoo, MI: The Upjohn Company; 1989. With permission of Pfizer Inc.
Table II.
Why Does Albuminuria Reflect the Status of the Circulation? Arterial and Glomerular Injury
| Increase | Adhesion Chemotaxis | |||||
|---|---|---|---|---|---|---|
| Cell | Proliferate | Migrate | Phenotype Change | Growth Factors | Matrix | Thrombosis |
| Endothelial | + | + | + | + | + | |
| Platelet | + | + | + | |||
| Macrophages | + | + | + | + | ||
| Smooth muscle | + | + | + | + | + | |
| Mesangial | + | + | + | + | + |
Several lines of evidence support a functionalstructural relationship between microalbuminuria and endothelial damage. In patients with systemic atherosclerosis, the transvascular escape rate of albumin is directly related to the amount of albumin lost in the urine. 13 This observation has been interpreted to indicate generalized "leakiness" to the macromolecules, which reflects widespread vascular damage. Furthermore, persons with elevated urinary albumin levels have impaired endothelial function (decreased flow‐associated dilation after brachial artery occlusion), even when diabetes and hypertension are absent. 14 In summary, microalbuminuria is an indicator of generalized endothelial injury, a hallmark of systemic atherosclerosis.
SHOULD MICROALBUMINURIA BE A THERAPEUTIC TARGET?
Based on the evidence relating microalbuminuria to CVD and renal risk and the availability of treatments to reduce albuminuria, it has been argued that the reduction of albuminuria should be a therapeutic target. Observational data indicate that people with type 2 diabetes who do not demonstrate a progressive increase in albuminuria are more likely to survive. 15 As reviewed elsewhere by Bakris 16 in this issue of The Journal of Clinical Hypertension, a number of studies have shown that a reduction in albuminuria, or overt proteinuria, correlates with improved renal survival and predicts better renal function.
Although less data are available for CVD, similar trends are emerging. When albuminuria is reduced by treatment, in particular with inhibition of the renin‐angiotensin system using a regimen based on angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, rates of major CVD events decrease in parallel. These observations have been made in various populations, including those with and without diabetes or hypertension. 17 , 18 Such data are suggestive that albuminuria can be used to assess a response to treatment and to optimize therapy based on individual responsiveness. At present, however, this concept should be viewed as a hypothesis that is not yet conclusive for CVD. To confirm this hypothesis, future studies should be performed with the reduction of albuminuria as the therapeutic target and CVD events as the main outcome. In the meantime, the measurement of albuminuria is clinically valuable to identify high‐risk individuals who should receive intensive risk factor management based on current treatment guidelines.
The therapy of microalbuminuria is focused on the management of hypertension and renin‐angiotensin‐system inhibition, as reviewed by Toto 19 and Weir 20 elsewhere in this issue, however, other therapies also show promise. These therapies include strict glycemic control in diabetes, lipid lowering with statins, the limitation of dietary protein, and aspirin. A similar multifactorial approach in people with type 2 diabetes and microalbuminuria reduced rates of major CVD and renal events by 50%–60%. 21 Thus the treatment of patients with microalbuminuria should include attention to the spectrum of major CVD risk factors. Whether albuminuria can be used to indicate responsiveness to multiple risk factor intervention, and whether it reflects a responsiveness to specific treatment components, remain to be determined. Nevertheless, high‐risk patients with microalbuminuria clearly benefit from strategies known to reduce CVD risk.
CONCLUSIONS
Microalbuminuria is a major, independent risk factor for CVD events in various groups including persons with diabetes or hypertension, the general population, and individuals with prevalent CVD. Microalbuminuria appears to be a stronger risk factor in men than in women, however, women with increased albuminuria levels are also at higher risk, and exogenous estrogen therapy raises the odds of microalbuminuria by about two‐fold. Microalbuminuria is an indicator of generalized endothelial injury, a hallmark of systemic atherosclerosis. Treatments that decrease albuminuria, particularly with agents that inhibit the renin‐angiotensin system, reduce CVD risk in various populations including individuals with and without diabetes or hypertension. Whether albuminuria should be a treatment target to reduce CVD risk, however, has not yet been proven. In the meantime, the measurement of albuminuria is clinically valuable to identify high‐risk individuals who should receive intensive risk factor management based on current treatment guidelines.
References
- 1. Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity‐onset diabetes. N Engl J Med. 1984;310:356–360. [DOI] [PubMed] [Google Scholar]
- 2. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 3. Jensen JS, Feldt‐Rasmussen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898–903. [DOI] [PubMed] [Google Scholar]
- 4. Hillege HL, Fidler V, Diercks GF, et al. Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. [DOI] [PubMed] [Google Scholar]
- 5. Romundstad S, Holmen J, Kvenild K, et al. Microalbuminuria and all‐cause mortality in 2,089 apparently healthy individuals: a 4.4 year follow‐up study. The Nord‐Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42:466–473. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. [DOI] [PubMed] [Google Scholar]
- 7. Hoehner CM, Greenlund KJ, Righ‐Najarian S, et al. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter‐Tribal Heart Project. J Am Soc Nephrol. 2002;13:1626–1634. [DOI] [PubMed] [Google Scholar]
- 8. Tuttle KR, Puhlman ME, Cooney SK, et al. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis. 1999;34:918–925. [DOI] [PubMed] [Google Scholar]
- 9. Gerstein HC, Mann JF, Yi Q, et al. HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 10. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . The JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 11. Roest M, Banga JD, Janssen WM, et al. Excessive urinary albumin levels are associated with future cardiovascular mortality in postmenopausal women. Circulation. 2001;103:3057–3061. [DOI] [PubMed] [Google Scholar]
- 12. Monster TB, Janssen WM, De Jong PE, et al. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161:2000–2005. [DOI] [PubMed] [Google Scholar]
- 13. Jensen JS. Renal and systemic transvascular albumin leakage in severe atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1324–1329. [DOI] [PubMed] [Google Scholar]
- 14. Clausen P, Jensen JS, Jensen G, et al. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–1874. [DOI] [PubMed] [Google Scholar]
- 15. Spoelstra‐de Man AM, Brouwer CB, Stehouwer CD, et al. Rapid progression of albumin excretion as an independent predictor of cardiovascular mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2001;24:2097–2101. [DOI] [PubMed] [Google Scholar]
- 16. Bakris GL. Implications of albuminuria on kidney disease progression. J Clin Hypertens (Greenwich). 2004;6(11 suppl 3):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brenner BM, Cooper ME, De Zeeuew D, et al. for the RENAAL Study Investigators. Effect of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 18. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 19. Toto RD. Microalbuminuria: definition, detection and clinical significance. J Clin Hypertens (Greenwich). 2004;6(11 suppl 3):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weir MR. Dietary salt, blood pressure, and microalbuminuria. J Clin Hypertens (Greenwich). 2004;6(11 suppl 3):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. [DOI] [PubMed] [Google Scholar]


