Abstract
This subgroup analysis of the Irbesartan/Hydrochlorothiazide (HCTZ) Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial evaluated the efficacy and safety of irbesartan/HCTZ fixed combinations in adults with uncontrolled systolic blood pressure (SBP) (140–159 mm Hg; 130–159 mm Hg for type 2 diabetes mellitus [T2DM]) after ≥4 weeks of antihypertensive monotherapy. Treatment was sequential: placebo (4–5 weeks), HCTZ 12.5 mg (2 weeks), irbesartan/HCTZ 150/12.5 mg (8 weeks), and irbesartan/HCTZ 300/25 mg (8 weeks). In the intent‐to‐treat analysis, mean change from baseline (end of placebo phase) off all previous therapy to Week 18 (study end) in T2DM patients (n=227) was −18.2±14.1 mm Hg for SBP (primary end point; p<0.001) and −8.7±8.2 mm Hg for diastolic blood pressure (p<0.001). Mean SBP/diastolic blood pressure changes in patients with the metabolic syndrome (n=345) were –21.0±14.3/−10.4±8.5 mm Hg (p<0.001). Overall, 56% (95% confidence interval, 49%–62%) of T2DM and 73% (95% confidence interval, 68%–77%) of metabolic syndrome patients achieved SBP goal (<140 mm Hg; <130 mm Hg for T2DM). Goal attainment rates were significantly higher among women with the metabolic syndrome than men. Treatments appeared to be well tolerated. Irbesartan/HCTZ fixed combinations achieved SBP goals in over half of the T2DM patients and nearly three quarters of patients with the metabolic syndrome, with SBP uncontrolled on antihypertensive monotherapy.
Hypertension is prevalent in individuals with the metabolic syndrome 1 and/or type 2 diabetes mellitus (T2DM). 1 , 2 The coexistence of hypertension in patients with T2DM increases the risk of macrovascular and microvascular complications, including stroke; coronary artery disease; peripheral vascular disease; retinopathy; nephropathy; and, possibly, neuropathy. 1 , 2 , 3 , 4 Individuals with hypertension as a component of their metabolic syndrome have a heightened risk of developing T2DM, coronary heart disease, stroke, and renal disease, as well as increased all‐cause and cardiovascular mortality rates. 4 Achieving blood pressure (BP) goal (<140/90 mm Hg for patients with uncomplicated hypertension; <130/80 mm Hg for patients with T2DM) reduces this associated morbidity and mortality. 5 , 6 Controlling BP has been shown to reduce the incidence of cardiovascular disease (CVD) and to delay the development of nephropathy in patients with T2DM. 5 , 7
Up to 75% of CVD and renal complications in patients with diabetes are attributable to hypertension. 2 However, a gap exists between recommended BP control and actual achievement of those goals in patients with diabetes and other complex forms of hypertension, such as patients with the metabolic syndrome. 7 For example, in 2002, data collected from urban academic medical centers revealed that only 3.2% of T2DM patients met the combined American Diabetes Association (ADA) goals for BP, low‐density lipoprotein cholesterol, and hemoglobin A1c <7%. 8 Further, only 26.7% met the target BP. In a more recent update of this database, only 28% of T2DM patients met the target BP of <130/80 mm Hg. 9 This is in general agreement with other recent reports of inadequate BP control in patients with T2DM. 10 , 11
Although a number of monotherapies and multidrug therapies are available for the treatment of hypertension, current guidelines provide evidence‐based recommendations for the use of specific antihypertensive agents in patients with T2DM. The ADA 5 and National Kidney Foundation 3 guidelines state that all patients with T2DM and hypertension should receive either an angiotensin‐converting enzyme inhibitor (ACEI) or an angiotensin II receptor blocker (ARB) as part of a treatment regimen. Most patients with hypertension will require at least two antihypertensive drugs to achieve BP goal. 1 , 2 Addition of a thiazide diuretic to ACEI or ARB treatment is a logical step and is currently recommended as part of initial antihypertensive therapy. 3 , 5 , 6 Use of combination products also reduces overall pill burden, an important consideration in patients with comorbidities. Furthermore, some combination products have improved tolerability compared with individual monotherapies. 2 , 6
In clinical trials, the concomitant administration of irbesartan and hydrochlorothiazide (HCTZ), as well as other ARBs or ACEIs either as individual components or in fixed‐dose combination, provides additive reductions in BP compared with the respective monotherapies in a wide range of patients and is well tolerated. 12 , 13 , 14 The current subgroup analysis of the Irbesartan/HCTZ Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial 15 , 16 aimed to evaluate the antihypertensive efficacy and safety of a fixed combination of irbesartan/HCTZ at a low dose (irbesartan/HCTZ 150/12.5 mg) and a high dose (irbesartan/HCTZ 300/25 mg) in patients with T2DM and/or the metabolic syndrome and systolic BP (SBP) that was uncontrolled on antihypertensive monotherapy.
Diabetic women have a substantially increased relative risk for CVD. 16 Over the past decade, ageadjusted heart disease mortality has declined 27% in nondiabetic women but has increased 23% in women with diabetes. 17 Whether this is related to the degree of SBP control remains unclear, because few data are available on the achievement of SBP goals in women. The current subanalysis of the INCLUSIVE population with T2DM or the metabolic syndrome also evaluated gender differences in the achievement of SBP goal.
METHODS
Study Design and Patients
Details of the trial patient population and study design have been published. 15 In summary, the INCLUSIVE trial was a multicenter, prospective, open‐label, single‐arm study conducted at 119 sites across the United States from July 2003 to August 2004. Participants were men and women, 18 years of age and older, with uncontrolled SBP at screening (140–159 mm Hg; 130–159 mm Hg for patients with T2DM) after ≥4 weeks of antihypertensive monotherapy. This subgroup analysis focused on patients with T2DM (defined as a fasting glucose ≥126 mg/dL and/or taking antidiabetic medication) and/or the metabolic syndrome (according to National Cholesterol Education Program [NCEP] criteria 2 , 18 ). Patients included in the metabolic syndrome subgroup could also have T2DM, since the NCEP definition of metabolic syndrome does not exclude diabetic patients. Excluded were patients with severe or secondary hypertension, significant concomitant disease, hypersensitivity to study medication, and those receiving insulin. 15 Of interest was the fact that a high percentage of patients (>50%) who had been unresponsive to monotherapy were receiving an ACEI or ARB.
Qualifying patients discontinued previous antihypertensive monotherapy and entered a four‐phase longitudinal treatment period with placebo (4–5 weeks), HCTZ 12.5 mg (2 weeks), a low‐dose irbesartan/HCTZ 150/12.5‐mg combination (8 weeks), and a high‐dose irbesartan/HCTZ 300/25‐mg combination (8 weeks), as described previously. 15 Seated BP was measured at trough (8 a.m.±2 hours) at each clinic visit using an automatic Omron device (HEM‐705CP model; Omron Healthcare Inc., Bannockburn, IL; variation error ±4 mm Hg). 19 BP was calculated from the mean of three readings obtained 2 minutes apart.
The Institutional Review Board/Ethics Committee of each participating site approved the study, and all patients gave written informed consent before enrollment.
Efficacy End Points
The primary efficacy end point was the mean change in SBP from placebo treatment end (baseline) to irbesartan/HCTZ 300/25‐mg treatment end (Week 18). Secondary end points were the mean change in diastolic BP (DBP) from baseline to Week 18, and mean changes in SBP and DBP from baseline to irbesartan/HCTZ 150/12.5‐mg treatment end (Week 10). Mean changes in SBP and DBP from baseline to HCTZ 12.5‐mg treatment end (Week 2) were also assessed. BP goal attainment rates were determined at Weeks 2, 10, and 18, according to the following criteria, which are consistent with current hypertension management guidelines 2 , 3 , 5 , 6 , 20 :
-
•
SBP goal: <130 mm Hg for patients with T2DM and <140 mm Hg for those with the metabolic syndrome without T2DM
-
•
DBP goal: <80 mm Hg for patients with T2DM and <90 mm Hg for those with the metabolic syndrome without T2DM.
Safety Evaluations
Adverse events (AEs) were monitored throughout the study. Clinically significant changes in physical examination or laboratory analyses of blood and urine samples were recorded as AEs.
Statistical Analyses
For the entire trial, a sample of 1042 patients was calculated to provide an estimate of the overall mean change in SBP from baseline to Week 18 to within 0.8 mm Hg of its true value, with 95% confidence and allowing for a 20% dropout rate. This sample size was also sufficient to provide an estimate of the mean change to Week 18 in SBP in the metabolic syndrome and T2DM subgroups to within 2.5 mm Hg of its true value with 95% confidence.
An intent‐to‐treat (ITT) analysis was conducted on efficacy data. This included patients with one or more valid SBP measurements after taking at least one dose of irbesartan/HCTZ 150/12.5 mg. Efficacy parameters were determined from the last observation carried forward for ITT patients withdrawing from the study before Week 18, including those who reached BP goal. Safety was evaluated for all patients taking at least one dose of placebo.
Mean and SDs, medians, and interquartile ranges were calculated for interval and ratio level variables. Mean changes in BP from baseline were tested using a paired t test for a normally distributed population. Alternate formulae from Fleiss 21 were used to calculate the upper and lower limits of the interval if point estimates were <0.1 or >0.9. Counts and percentages were calculated for categoric variables. An analysis of variance (ANOVA) was employed to test hypotheses of differences between gender groups in mean changes in SBP and DBP, and associated 95% confidence intervals (CIs) were constructed for difference parameter estimates, including those of gender comparisons. If the parameter estimates were determined to be non‐normally distributed, the Wilcoxon‐Mann‐Whitney test was employed to test hypotheses related to changes in SBP and DBP. The Shapiro‐Wilk test was used in combination with graphic methods to assess large violations of normality. The Fisher exact test was employed for statistical tests involving goal attainment rates, and 95% CIs with Yates' continuity correction were constructed around gender differences between goal attainment rates.
RESULTS
Patient Population
A total of 1005 patients, of whom 295 had T2DM and 388 had the metabolic syndrome, entered the placebo treatment phase (safety population). Subsequently, 844 patients were enrolled into the HCTZ 12.5‐mg treatment phase. Of these, 254 patients (30%) had T2DM and 386 (46%) had the metabolic syndrome; 177 (21%) patients in each of these groups had concomitant T2DM and metabolic syndrome (Table I). Overall, 190 patients with T2DM and 295 with the metabolic syndrome completed the study. The most common reason for discontinuation was that patients did not meet BP qualification criteria at the start of a treatment period (17% of T2DM patients; 12% of metabolic syndrome patients), the vast majority because their BP was below rather than above the predefined limits. AEs accounted for 17.1% (n=18) of discontinuations among patients with T2DM and 25.8% (n=24) among patients with the metabolic syndrome.
Table I.
Patient Demographics at Baseline
| With T2DM (n=254)* | Without T2DM (n=590) | With the Metabolic Syndrome (n=386)** | Without the Metabolic Syndrome (n=449)** | With T2DM and the Metabolic Syndrome (n=177) | |
|---|---|---|---|---|---|
| Mean age (yr) | 58.2 | 56.9 | 55.3 | 59.0 | 56.7 |
| Women (n [%]) | 112 (44) | 324 (55) | 213 (55) | 217 (48) | 87 (49) |
| Men (n [%]) | 142 (56) | 266 (45) | 173 (45) | 232 (52) | 90 (51) |
| Race/ethnic group (n [%])† | |||||
| Caucasian | 153 (60) | 362 (61) | 246 (64) | 262 (58) | 112 (63) |
| African American | 48 (19) | 143 (24) | 72 (19) | 117 (26) | 31 (18) |
| Hispanic/Latino | 50 (20) | 69 (12) | 64 (17) | 55 (12) | 33 (19) |
| Other | 3 (1) | 18 (3) | 6 (2) | 15 (3) | KD |
| Previous antihypertensive monotherapy (n [%]) | |||||
| β Blocker | 28 (11) | 69 (12) | 51 (13) | 46 (10) | 21 (12) |
| α Blocker | 4 (2) | 7 (1) | 4 (1) | 7 (2) | 2 (1) |
| Calcium channel blocker | 35 (14) | 133 (23) | 65 (17) | 101 (22) | 26 (15) |
| Angiotensin receptor blocker | 38 (15) | 128 (22) | 65 (17) | 99 (22) | 25 (14) |
| Angiotensin‐converting enzyme inhibitor | 118 (46) | 165 (28) | 141 (37) | 139 (31) | 84 (47) |
| Diuretic | 29 (11) | 85 (14) | 59 (15) | 53 (12) | 18 (10) |
| Other | 4 (2) | 9 (1) | 5 (1) | 8 (2) | 3 (2) |
| *Includes 177 patients with concomitant type 2 diabetes mellitus (T2DM) and the metabolic syndrome; **metabolic syndrome status of nine patients unknown; †patients could self‐identify into more than one race/ethnic group | |||||
Patients With T2DM
Mean Changes in BP From Baseline. Mean changes in SBP and DBP from baseline to the end of each treatment period were calculated separately for patients with and without T2DM in the ITT population (Table II).
Table II.
Mean Changes in Blood Pressure (mm Hg) From Baseline (Intent‐to‐Treat [ITT] Analysis)
| Patients With T2DM* | Baseline (Week 0) | HCTZ 12.5 mg (Week 2) | Irbesartan/HCTZ 150/12.5 mg (Week 10) | Irbesartan/HCTZ 300/25 mg (Week 18)** |
|---|---|---|---|---|
| No. in ITT population | 227 | 227 | 211 | 227 |
| SBP | ||||
| Mean ± SD at visit | 152.8±11.2 | 150.1±11.1 | 139.9±12.4 | 134.6±14.9 |
| Change from baseline (mean ± SD) | — | −2.7+10.9 | −13.2+12.5 | −18.2+14.1 |
| 95% CI for change from baseline | — | −4.1 to −1.3 | −14.9 to −11.5 | −20.0 to −16.4 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| DBP | ||||
| Mean ± SD at visit | 89.6±8.9 | 88.5±9.2 | 83.9±9.0 | 80.8±10.1 |
| Change from baseline (mean ± SD) | — | −1.1±6.7 | −6.1±7.6 | −8.7±8.2 |
| 95% CI for change from baseline | — | −2.0 to −0.2 | −7.1 to −5.0 | −9.8 to −7.7 |
| p Value for change from baseline | — | 0.015 | <0.001 | <0.001 |
| Patients Without T2DM | ||||
| No. in ITT population | 509 | 509 | 472 | 509 |
| SBP | ||||
| Mean ± SD at visit | 155.2±9.6 | 152.2±10.0 | 139.5±12.5 | 132.2±13.2 |
| Change from baseline (mean ± SD) | — | −3.0±10.3 | −15.9±12.5 | −23.0±14.2 |
| 95% CI for change from baseline | — | −3.9 to −2.1 | −17.0 to −14.8 | −24.2 to −21.7 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| DBP | ||||
| Mean ± SD at visit | 92.3±8.6 | 90.9±8.9 | 84.9±8.7 | 81.2±9.5 |
| Change from baseline (mean ± SD) | — | −1.4±6.9 | −7.8±8.2 | −11.1±8.8 |
| 95% CI for change from baseline | — | −2.0 to −0.8 | −8.5 to −7.0 | −11.9 to −10.4 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| Patients With the Metabolic Syndrome* | ||||
| No. in ITT population | 345 | 345 | 327 | 345 |
| SBP | ||||
| Mean ± SD at visit | 154.6±10.6 | 151.4±10.3 | 139.7±12.5 | 133.6±14.1 |
| Change from baseline (mean ± SD) | — | −3.2±10.4 | −15.0±12.8 | −21.0±14.3 |
| 95% CI for change from baseline | — | −4.3 to −2.1 | −16.4 to −13.6 | −22.5 to −19.5 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| DBP | ||||
| Mean ± SD at visit | 93.1±7.8 | 91.4±8.5 | 85.5±8.5 | 82.7±9.4 |
| Change from baseline (mean ± SD) | — | −1.8±6.7 | −7.8±7.9 | −10.4±8.5 |
| 95% CI for change from baseline | — | −2.5 to −1.1 | −8.6 to −6.9 | −11.3 to −9.5 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| Patients Without the Metabolic Syndrome | ||||
| No. in ITT population | 384 | 384 | 349 | 384 |
| SBP | ||||
| Mean ± SD at visit | 154.4±9.9 | 151.8±10.4 | 139.6±12.5 | 132.4±13.6 |
| Change from baseline (mean ± SD) | — | −2.7±10.6 | −15.3±12.3 | −22.1 ± 14.4 |
| 95% CI for change from baseline | — | −3.7 to −1.6 | −16.6 to −14.0 | −23.5 to −20.6 |
| p Value for change from baseline | — | <0.001 | <0.001 | <0.001 |
| DBP | ||||
| Mean ± SD at visit | 90.0±9.3 | 89.1±9.4 | 83.7±9.1 | 79.7±9.9 |
| Change from baseline (mean ± SD) | — | −0.9±6.9 | −6.8±8.2 | −10.3±8.8 |
| 95% CI for change from baseline | — | −1.6 to −0.2 | −7.7 to −5.9 | −11.2 to −9.4 |
| p Value for change from baseline | — | 0.01 | <0.001 | <0.001 |
| HCTZ=hydrochlorothiazide; SBP=systolic blood pressure; CI=confidence interval; DBP=diastolic blood pressure; *indudes 157 patients with concomitant type 2 diabetes mellitus (T2DM) and the metabolic syndrome; **last observation carried forward for Week 18 data | ||||
The mean change in SBP from baseline to Week 18 (primary end point) was −18.2±14.1 mm Hg (95% CI, −20.0 to −16.4 mm Hg; p<0.001) for patients with T2DM and −23.0±14.2 mm Hg (95% CI, −24.2 to −21.7 mm Hg; p<0.001) for patients without T2DM. Final mean SBP was 134.6±14.9 mm Hg among patients with T2DM and 132.2±13.2 mm Hg among those without T2DM.
Statistically significant reductions in DBP were also observed. At Week 18, the mean change in DBP from baseline was −8.7±8.2 mm Hg (95% CI, −9.8 to −7.7 mm Hg; p<0.001) for patients with T2DM and −11.1±8.8 mm Hg (95% CI, −11.9 to −10.4 mm Hg; p<0.001) for those without T2DM. Mean DBP at study end was similar for patients with and without T2DM (80.8±10.1 mm Hg and 81.2±9.5 mm Hg, respectively).
Goal Attainment Rates. The SBP and DBP goal attainment rates were determined for patients with and without T2DM (Table III). In keeping with hypertension management guidelines, BP goals for patients with T2DM (<130/80 mm Hg) were more stringent than for patients without T2DM (<140/90 mm Hg). At study end, 56% (95% CI, 49%–62%) of patients with T2DM reached SBP goal, 63% (95% CI, 56%–69%) reached DBP goal, and 40% (95% CI, 34%–46%) reached dual SBP and DBP goal. Goal attainment rates for patients without T2DM were 87% (95% CI, 84%–90%) for SBP, 92% (95% CI, 90%–94%) for DBP, and 82% (95% CI, 78%–85%) for both SBP and DBP.
Table III.
Blood Pressure (BP) Goal* Attainment Rates (n [%]) (Intent‐to‐Treat Analysis)
| With T2DM** (n=227) | Without T2DM (n=509) | With the Metabolic Syndrome** (n=345) | Without the Metabolic Syndrome (n=384) | With T2DM and the Metabolic Syndrome (n=157) | |
|---|---|---|---|---|---|
| SBP goal | |||||
| Baseline to Week 2 | 2 (1) | 19 (4) | 2 (1) | 19 (5) | 1 (1) |
| Baseline to Week 10 | 67 (30) | 344 (68) | 169 (49) | 238 (62) | 41 (26) |
| Baseline to Week 18 | 127 (56) | 442 (87) | 251 (73) | 312 (81) | 90 (57) |
| 95% CI for baseline to Week 18 (%) | 49–62 | 84–90 | 68–77 | 77–85 | 50–65 |
| DBP goal | |||||
| Baseline to Week 2 | 43 (19) | 223 (44) | 86 (25) | 178 (46) | 25 (16) |
| Baseline to Week 10 | 95 (42) | 424 (83) | 209 (61) | 304 (79) | 57 (36) |
| Baseline to Week 18 | 142 (63) | 468 (92) | 264 (77) | 339 (88) | 92 (59) |
| 95% CI for baseline to Week 18 (%) | 56–69 | 90–94 | 72–81 | 85–92 | 51–66 |
| SBP and DBP goal | |||||
| Baseline to Week 2 | 2 (1) | 13 (3) | 2 (1) | 13 (3) | 1 (1) |
| Baseline to Week 10 | 44 (19) | 310 (61) | 137 (40) | 213 (55) | 26 (17) |
| Baseline to Week 18 | 91 (40) | 416 (82) | 211 (61) | 290 (76) | 61 (39) |
| 95% CI for baseline to Week 18 (%) | 34–46 | 78–85 | 56–66 | 71–80 | 31–47 |
| Week 2=end of hydrochlorothiazide (HCTZ) 12.5‐mg treatment; Week 10=end of irbesartan/HCTZ 150/12.5‐mg treatment; Week 18=end of irbesartan/HCTZ 300/25‐mg treatment; CI=confldence interval; *systolic BP (SBP) goal: <140 mm Hg; <130 mm Hg for patients with type 2 diabetes mellitus (T2DM); diastolic BP (DBP) goal: <90 mm Hg; <80 mm Hg for patients with T2DM; **includes 157 patients with concomitant T2DM and the metabolic syndrome | |||||
Patients With the Metabolic Syndrome
Mean Changes in BP From Baseline. Mean changes in BP from baseline to Weeks 2, 10, and 18 were calculated separately for patients with and without the metabolic syndrome in the ITT population (Table II).
A mean change in SBP from baseline to Week 18 of −21.0±14.3 mm Hg (95% CI, −22.5 to −19.5 mm Hg; p<0.001) was observed for patients with the metabolic syndrome and −22.1±14.4 mm Hg (95% CI, −23.5 to −20.6 mm Hg; p<0.001) for patients without the metabolic syndrome. Mean SBP for Week 18 among patients with the metabolic syndrome was 133.6±14.1 mm Hg and 132.4±13.6 mm Hg among those without the metabolic syndrome.
The mean change in DBP was −10.4±8.5 mm Hg (95% CI, −11.3 to −9.5 mm Hg; p<0.001) and −10.3±8.8 mm Hg (95% CI, −11.2 to −9.4 mm Hg; p<0.001) for patients with and without the metabolic syndrome, respectively (Table II). Mean DBP for Week 18 was 82.7±9.4 mm Hg for patients with the metabolic syndrome vs. 79.7±9.9 mm Hg for patients without the metabolic syndrome.
Goal Attainment Rates. BP goal attainment rates for Week 18 were determined for patients with and without the metabolic syndrome (Table III). The results were further analyzed based on T2DM and gender status.
Among patients with the metabolic syndrome, 73% (95% CI, 68%–77%) achieved SBP goal, 77% (95% CI, 72%–81%) achieved DBP goal, and 61% (95% CI, 56%–66%) achieved dual SBP/DBP goal. Goal attainment rates among patients without the metabolic syndrome were 81% (95% CI, 77%–85%) for SBP, 88% (95% CI, 85%–92%) for DBP, and 76% (95% CI, 71%–80%) for SBP/DBP.
For patients in the ITT population with both the metabolic syndrome and T2DM (n=157), 57% (95% CI, 50%–65%) reached SBP goal, 59% (95% CI, 51%–66%) reached DBP goal, and 39% (95% CI, 31%–47%) reached dual SBP/DBP goal.
Results According to Gender
Of the patients enrolled, 44% were women with T2DM and 55% were women with the metabolic syndrome. Among patients with T2DM, SBP at baseline was similar between men and women (152.9±11.3 mm Hg vs. 152.7±11.1 mm Hg, respectively), as was DBP (90.0±9.0 mm Hg vs. 89.0±8.8 mm Hg, respectively). For the subgroup with the metabolic syndrome, baseline BP was marginally higher in men than women (155.3±11.0/94.1±7.3 mm Hg vs. 154.0±10.2/92.2±8.2 mm Hg).
The ANOVA examining differences in efficacy end points between genders showed no statistically significant difference between women and men with T2DM in mean SBP change from baseline to Week 18 (women, −19.0±15.1 mm Hg; men, −17.6±13.3 mm Hg; difference, −1.4 mm Hg; 95% CI, −5.1 to 2.4 mm Hg; p=0.47) or mean DBP change from baseline to Week 18 (women, −8.4±8.4 mm Hg; men, −8.9±8.0 mm Hg; difference, 0.53 mm Hg; 95% CI, −1.6 to 2.7 mm Hg, p=0.63). Similarly, no significant difference was found between women and men with the metabolic syndrome with regard to the mean SBP change (women, −22.1±14.6 mm Hg; men, −19.7±14.0 mm Hg; difference, −2.5 mm Hg; 95% CI, −5.5 to 0.6 mm Hg; p=0.11) or mean DBP change (women, −10.5±8.8 mm Hg; men, −10.4±8.2 mm Hg; difference, −0.12 mm Hg; 95% CI, −1.9 to 1.7 mm Hg; p=0.90) from baseline to Week 18.
The percentages of women and men with T2DM achieving SBP goal (difference, 6.7%; 95% CI, −7.2% to 20.6%; p=0.35), DBP goal (difference, 9.6%; 95% CI, −3.9% to 23.0%; p=0.17), and dual SBP/DBP goal (difference, 11.0%; 95% CI, −2.8% to 24.8%; p=0.10) were also statistically comparable (Figure). In contrast, significantly more women than men with the metabolic syndrome achieved SBP, DBP, and dual SBP/DBP goals at Week 18 (Figure).
Figure.
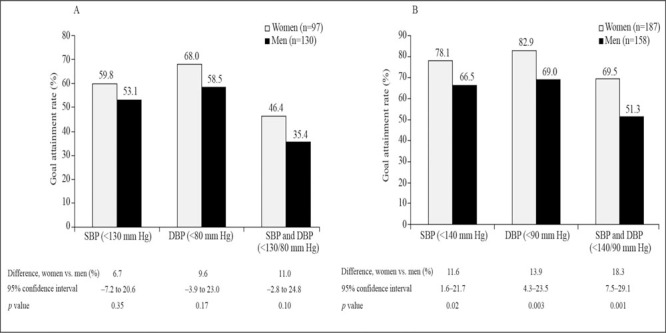
Systolic blood pressure (SBP) and diastolic blood pressure (DBF) goal attainment rates at Week 18 for men vs. women with A) type 2 diabetes mellitus or B) the metabolic syndrome (intent‐to‐treat analysis)
Safety
All 1005 patients entering the placebo run‐in period underwent safety evaluations. All treatments were well tolerated, irrespective of subgroup, with most AEs being of mild or moderate intensity, transient in duration, and in keeping with label indications. Overall, 60% (178 of 295) of patients in the safety population with T2DM experienced an AE: 24% during placebo treatment, 18% during HCTZ 12.5‐mg treatment, 28% during irbesartan/HCTZ 150/12.5‐mg treatment, and 27% during irbesartan/HCTZ 300/25‐mg treatment. In addition, AEs were reported by 255 of 388 (66%) of metabolic syndrome patients: 27% while on placebo, 18% on HCTZ 12.5 mg, 29% on irbesartan/HCTZ 150/12.5 mg, and 26% on irbesartan/HCTZ 300/25 mg. Dizziness was the most common AE experienced overall, occurring in 6% of T2DM and 5% of metabolic syndrome patients. Interestingly, only one AE of hyperkalemia occurred during the irbesartan/HCTZ, 300/25‐mg treatment period. The relationship of one serious AE (hypotension) to study medication (irbesartan/HCTZ 150/12.5 mg) was considered “probable”; all other serious AEs were judged unrelated to study medication. For comparison, the AE rate in the total population was 55% (551 of 1005). The summary of AEs is given in Table IV and Table V.
Table IV.
Adverse Events Occurring in ≥2% of Patients During Treatment With Either Irbesartan/HCTZ Combination
| Patients With T2DM | During Placebo | During HCTZ 12.5 mg | During Irbesartan/ HCTZ 150/12.5 mg | During Irbesartan/ HCTZ 300/25 mg |
|---|---|---|---|---|
| Safety population (n) | 295 | 254 | 243 | 215 |
| Any adverse event (n [%])* | 72 (24) | 46 (18) | 69 (28) | 59 (27) |
| Adverse event (n [%]) | ||||
| Dizziness | 2 (1) | 4 (2) | 5 (2) | 6 (3) |
| Nasopharyngitis | 5 (2) | 4 (2) | 2 (1) | 5 (2) |
| Upper respiratory tract infection | 9 (3) | 1 (0) | 5 (2) | 0 |
| Influenza | 3 (1) | 1 (0) | 8 (3) | 0 |
| Bronchitis | 0 | 2 (1) | 1 (0) | 5 (2) |
| Patients With the Metabolic Syndrome | ||||
| Safety population (n) | 388 | 386 | 366 | 329 |
| Any adverse event (n [%])* | 105 (27) | 68 (18) | 107 (29) | 85 (26) |
| Adverse event (n [%]) Dizziness | 4 (1) | 4 (1) | 10 (3) | 6 (2) |
| HCTZ=hydrochlorothiazide; T2DM=type 2 diabetes mellitus; *some patients may have experienced more than one adverse event | ||||
Table V.
Serious Adverse Events (SAEs) in Patients With T2DM and/or the Metabolic Syndrome
| Treatment Phase | SAEs | Subgroup (T2DM and/or the Metabolic Syndrome) | Relationship to Study Treatment |
|---|---|---|---|
| Prestudy (screening) | Chronic lymphocytic leukemia | T2DM and metabolic syndrome | Unrelated |
| During placebo | Prostate infection/prostatitis/nausea/vomiting | T2DM and metabolic syndrome | Unrelated |
| During HCTZ 12.5 mg | Colitis | T2DM and metabolic syndrome | Unrelated |
| Carotid stenosis repair | T2DM | Unrelated | |
| Leg cellulitis/T2DM | T2DM and metabolic syndrome | Unrelated | |
| Acute myocardial infarction | Metabolic syndrome | Unrelated | |
| During Irbesartan/HCTZ 150/12.5 mg | Facial cellulitis | Metabolic syndrome | Unrelated |
| Bilateral diabetic lumbosacral plexitis | T2DM and metabolic syndrome | Unrelated | |
| Acute abdominal pain/gastroenteritis/colitis | T2DM and metabolic syndrome | Unrelated | |
| Foot fracture and cellulitis following an | T2DM and metabolic syndrome | Unrelated | |
| automobile accident | |||
| Hypotension | T2DM | Probably related | |
| During Irbesartan/HCTZ 300/25 mg | Carcinoid tumor of the distal ileum | Metabolic syndrome | Unrelated |
| T2DM/hyperlipidemia | Metabolic syndrome | Unrelated | |
| Acute abdominal pain/gallstone | T2DM and metabolic syndrome | Unrelated | |
| Rectal bleeding/benign rectal polyp/Crohn's disease | T2DM and metabolic syndrome | Unrelated | |
| Left flank abdominal pain/pancytopenia | T2DM and metabolic syndrome | Unrelated | |
| T2DM=type 2 diabetes mellitus; HCTZ=hydrochlorothiazide | |||
DISCUSSION
The achievement of BP goals in the current study population was generally better than previously observed in some studies, especially in a diabetic cohort. Overall, 56% of subjects with T2DM and 73% of subjects with the metabolic syndrome (with or without concomitant T2DM) achieved their SBP goal (<130 mm Hg for patients with T2DM; <140 mm Hg for those without T2DM) after up‐titration to the higher doses of the ARB and HCTZ. Among subjects with T2DM, the dual SBP/DBP goal attainment rate (40%) was considerably better than for diabetic patients in other trials 22 , 23 , 24 , 25 , 26 , 27 , 28 and in practice‐based settings (26.7%, 28%, and 35.8%, respectively). 8 , 9 , 10 Further, the fact that these goals were achieved in the majority of both groups of hypertension patients may be of particular clinical relevance given the increasing evidence that early aggressive control is important in determining CVD outcomes.
Complex hypertension is a term currently given to hypertensive patients with clinical evidence of CVD or target organ damage or those at high risk for CVD, such as patients with diabetes or the metabolic syndrome. 7 , 29 , 30 Some reports suggest that the risk of CVD among patients with diabetes is equivalent to that of patients who have already had a coronary heart disease event, 2 , 31 although other reports suggest that the CVD risk is lower among individuals with diabetes. 32 The metabolic syndrome patient has a four‐fold increased risk of fatal coronary heart disease and a five‐ to ninefold risk of developing diabetes. 6 Furthermore, cardiometabolic risk factors and CVD appear to be concurrent across a spectrum of nondiabetic blood glucose readings. 33 Thus, the current subanalysis of the INCLUSIVE trial demonstrates the ability of a fixed‐dose combination of an ARB/HCTZ to achieve BP goals in over half of diabetic patients and three quarters of patients with the metabolic syndrome whose SBP was uncontrolled on antihypertensive monotherapy. It is likely that these results can be obtained with other ARB/HCTZ combinations. However, there are no presently available data on other combinations. These control data are in concert with several small trials and observational studies of other ARB/diuretic combinations, as recently reviewed. 34
There is a wide gap between recommended BP goals and achievement of these goals in patients with complex hypertension in clinical practice. Why is it so difficult to achieve these goals? One issue relates to the fact that it is more difficult to lower BP in patients with the metabolic syndrome and diabetes. These patients are typically overweight, may have some degree of renal compromise, have less vascular compliance, and may have disproportionate elevations in SBP. 1 , 2 , 3 , 4 , 5 , 7 , 8 , 9 Clinical inertia may also be a factor, with health care providers accepting inadequate BP control, especially SBP. 35 , 36 Another issue may be the more aggressive goals that have been recommended, i.e., 130/80 mm Hg in patients with diabetes. Finally, there is the problem that relates to the use of antihypertensive monotherapy, or sub optimal antihypertensive regimens. The use of combination antihypertensive agents may be one way to help physicians overcome this inertia and to improve patient adherence.
The major reason for lack of attainment of BP goals is the inability to meet SBP goals. 7 , 8 , 9 , 10 The importance of attaining adequate SBP reduction is becoming increasingly apparent. Indeed, in patients with stage 1 hypertension, a 12‐mm Hg decrease in SBP over 10 years prevents one death for every 12 patients treated who have CVD or diabetes mellitus. 37 , 38 Even small reductions in SBP of 3–5 mm Hg may result in definite reductions in CVD events such as heart failure and stroke. As noted, a number of studies have utilized combination therapy with different ARBs.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 6 recommends the use of two agents, or a combination of two agents from different classes, in patients with BP levels >20 mm Hg above goal SBP (>160 mm Hg) or 10 mm Hg above goal DBP (100 mm Hg). One of the drugs in the combination should be a diuretic. In addition, this committee advocates using an ACEI or an ARB in patients with diabetes and/or renal disease. 6 The results of this trial demonstrate that the combination of one of the ARB/HCTZ combinations (irbesartan/HCTZ in a dose of 300/25 mg daily) controls SBP in a substantial proportion of a heterogeneous group of hypertensive patients, including those with T2DM or the metabolic syndrome who were not previously controlled on monotherapy. 18 Side effects were not troublesome, presumably due to the fact that the components of this combination, irbesartan and diuretics, have a good tolerability profile in the doses used in this study. Thus, these data indicate that many patients, including complex hypertensive patients, with inadequate SBP control can reach current SBP goal levels. The current study also demonstrated that women with T2DM or the metabolic syndrome respond at least as well as men with regard to SBP goal attainment. This is an important observation given the high CVD risk in women with these complex forms of hypertension.
Appendix
In addition to the authors, the INCLUSIVE Investigators are as follows: L. Altschul, Z. Ansari, P. Arcuri, H. Bays, H. Biermann, N. Bittar, J. Bloom, K. Bordenave, D. Brautigam, P. Buchanan, A. Carr, J. Champlin, D. Cheung, S. Chrysant, J. Clower III, G. Collins, M. DeBruin, E. Denoia, V. Desai, M. Dewan, M. El Shahawy, A. Elkind, R.D. Ferrera, J. Fidelholtz, N. Fraser, L. Gidday, L. Gilderman, R. Ginsburg, E. Hare, D. Hassman, M. Heitbrink, M. Henriquez, J. Herron, G. Hilliard, P. Hughes, H. Ibrahim, T. Isakov, W. Jacobs, J. Jernigan, L. Judy, D. Kereiakes, B. Kerzner, L. Koehler, M. Koren, M. Kozinn, E. Kunst, G. Lankin, A. Lewin, T. Littlejohn III, B. Lubin, R. Madder, R. Marple, J. Mersey, F. Messerli, N. Messina III, M. Mollen, P. Narayan, S. Nesbitt, S. Ong, S. Oparil, R. Owens, C. Patel, M. Peshimam, F. Pettyjohn, A. Phillips, I. Plisco, M. Pohl, J. Quesada, G. Raad, B. Rankin, D. Riff, S. Rosansky, E. Roth, G. Ruoff, J. Sandoval, J. Saponaro, S. Shahzad, K. Sheehan, T. Sherraden, D. Sica, J. Siemienczuk, N. Singh, G. Smith, W. Smith, J. Soufar, W. Starling, K. Stone, C. Strout, R. Strzinek, D. Sugimoto, G. Szenkiel, R. Tidman, M. Tonkon, M.S. Touger, H. Underwood, C. Uy, J. Wadleigh, V. Wassily, J. Wayne, V. Weber, R. Weiss, N. Winer, D. Young, J. Zebrack.
Acknowledgement and discloure: The development of this manuscript was made possible with editorial assistance provided by Reagan Blyth, who provided medical writing services on behalf of the Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership. The clinical study reported in this publication was sponsored by the Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership. The sponsor provided funding and in‐kind support for the clinical trial and the publication. The sponsor participated in discussions with the INCLUSIVE Trial Steering Committee regarding study design and protocol development, and provided logistic support during the study. Integrium, a contract research organization under contract with the sponsor, monitored the study and site management. PAREXEL, a contract research organization under contract with the sponsor, conducted data entry and performed statistical analysis of the study data.
Author disclosures: James R. Sowers, MD, has grants from the NIH (R01‐HL‐63904), the VA Merit Reward, AstraZeneca, and Novartis and is on the Speaker's Bureau for Bristol‐Myers Squibb, Merck & Co, and Novartis. Joel M. Neutel, MD, provides speaking and consulting services for Biovail, Boehringer Ingelheim, Novartis, Bristol‐Myers Squibb, Pfizer, Reliant, Sanofi‐Aventis, and Sankyo. He also has an interest in and provides consulting services to Integrium, the clinical research organization that provided clinical management for this trial. Elijah Saunders, MD, provides speaking and consulting services for Abbott, Bristol‐Myers Squibb, Novartis, Pfizer, Sanofi‐Aventis, and Wyeth/Monarch. George L. Bakris, MD, is a consultant/Speaker's Bureau member/advisory board member for AstraZeneca, AusAm, Abbott, Boehringer Ingelheim, Bristol‐Myers SquibblSanofi‐Aventis, GlaxoSmithKline, Merck & Co, Novartis, Lilly, and Walgreens (formulary committee) and receives grants from NIH (NIDDK/NHLBI), Abbott, Boehringer Ingelheim, GlaxoSmithKline, Merck & Co, Novartis, and hilly. William C. Cushman, MD, receives grant/research support from Abbott, AstraZeneca, Biovail, Boehringer Ingelheim, Bristol‐Myers Squibb, King, Kos, Novartis, and Sanofi‐Aventis and provides consulting services to Abbott, Biovail, Boehringer Ingelheim, Bristol‐Myers Squibb, First Horizon, Forest, Novartis, Pfizer, Reddy, Sankyo, and Sanofi‐Aventis. Keith C. Ferdinand, MD, has received grants from AstraZeneca, Pfizer, Merck & Co, Novartis, Bristol‐Myers Squibb, and NitroMed. Elizabeth O. Ofili, MD, is supported by NIH grants #5P20RR11104 (Research Centers at Minority Institutions) and #5U54RR14758 (Center of Clinical Research Excellence) and the Medtronic Foundation. Dr Ofili has served as a consultant for Bristol‐Myers Squibb, NitroMed, Novartis, and Sanofi‐Aventis. Michael A. Weber, MD, provides speaking and consulting services for Boehringer Ingelheim, Novartis, Bristol‐Myers Squibb, Merck & Co, Pfizer, Sanofi‐Aventis, and Sankyo. He also has an interest in and provides consulting services to Integrium.
References
- 1. Sowers JR, Haffner S. Treatment of cardiovascular and renal risk factors in the diabetic hypertensive. Hypertension. 2002;40:781–788. [DOI] [PubMed] [Google Scholar]
- 2. Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004;164:1850–1857. [DOI] [PubMed] [Google Scholar]
- 3. National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Kidney Disease Outcomes Quality Initiative (K/DOQI). Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 4. Ninomiya JK, L'ltalien G, Criqui MH, et al. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 6. The Seventh Report of the Joint National Committee on Prevention. Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears in JAMA. 2003;290:197]. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Mugo MN, Sowers JR. Early and aggressive treatment of complex hypertension. J Clin Hypertens (Greenwich). 2005;7:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McFarlane SI, Jacober SJ, Winer N, et al. Control of cardiovascular risk factors in patients with diabetes and hypertension at urban academic medical centers. Diabetes Care. 2002;25:718–723. [DOI] [PubMed] [Google Scholar]
- 9. McFarlane SI, Castro J, Kaur J, et al. Control of blood pressure and other cardiovascular risk factors at different practice settings: outcomes of care provided to diabetic women compared to men. J Clin Hypertens (Greenwich). 2005;7:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. [DOI] [PubMed] [Google Scholar]
- 11. Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 13. Coca A, Calvo C, Sobrino J, et al. Once‐daily fixed‐combi nation irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003;25:2849–2864. [DOI] [PubMed] [Google Scholar]
- 14. Howe P, Phillips P, Saini R, et al. The antihypertensive efficacy of the combination of irbesartan and hydrochlo rothiazide assessed by 24‐hour ambulatory blood pressure monitoring. Irbesartan Multicenter Study Group. Clin Exp Hypertens. 1999;21:1373–1396. [DOI] [PubMed] [Google Scholar]
- 15. Neutel JM, Saunders E, Bakris GL, et al. The efficacy and safety of low‐ and high‐dose fixed combinations of irbesartan/HCTZ in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sowers JR. Diabetes mellitus and cardiovascular disease in women. Arch Intern Med. 1998;158:617–621. [DOI] [PubMed] [Google Scholar]
- 17. McFarlane SI, Sowers JR. Gender disparities in the control of cardiovascular risk factors in people with diabetes. J Clin Hypertens (Greenwich). 2005;7:383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 19. O'Brien E, Mee F, Atkins N, et al. Evaluation of three devices for self‐measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM‐705CP, Philips HP5332, and Nissei DS‐175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- 20. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 21. Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley & Sons; 1981. [Google Scholar]
- 22. Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hyper tension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. [DOI] [PubMed] [Google Scholar]
- 23. Niskanen L, Hedner T, Hansson L, et al. Reduced car diovascular morbidity and mortality in hypertensive dia betic patients on first‐line therapy with an ACE inhibitor compared with a diuretic/beta‐blocker‐based treatment regimen: a subanalysis of the Captopril Prevention Project. Diabetes Care. 2001;24:2091–2096. [DOI] [PubMed] [Google Scholar]
- 24. UK Prospective Diabetes Study Group . Tight blood pres sure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 25. Singer GM, Izhar M, Black HR. Guidelines for hypertension: are quality‐assurance measures on target? Hypertension. 2004;43:198–202. [DOI] [PubMed] [Google Scholar]
- 26. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT [published corrections appear in JAMA. 2003;289:178 and JAMA. 2004;291:2196]). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 27. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 28. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hyper tensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 29. Bakris GL, Gaxiola E, Messerli FH, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR‐Trandolapril study. Hypertension. 2004;44:637–642. [DOI] [PubMed] [Google Scholar]
- 30. Brooks CS, Sowers JR. Importance of achieving lower blood pressure in hypertensive patients with diabetes. Hypertension. 2004;44:614–615. [DOI] [PubMed] [Google Scholar]
- 31. Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 32. Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ. 2002;324:939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into can cer in Norfolk. Ann Intern Med. 2004;141:413–420. [DOI] [PubMed] [Google Scholar]
- 34. Kjeldsen SE, Os I, Hoieggen A, et al. Fixed‐dose combinations in the management of hypertension: defining the place of angiotensin receptor antagonists and hydrochlorothiazide. Am J Cardiovasc Drugs. 2005;5:17–22. [DOI] [PubMed] [Google Scholar]
- 35. Berlowitz DR, Ash AS, Hickey EC, et al. Hypertension management in patients with diabetes: the need for more aggressive therapy. Diabetes Care. 2003;26:355–359. [DOI] [PubMed] [Google Scholar]
- 36. Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–1963. [DOI] [PubMed] [Google Scholar]
- 37. Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens. 2003;21:1055–1076. [DOI] [PubMed] [Google Scholar]
- 38. White WB. Update on the drug treatment of hypertension in patients with cardiovascular disease. Am J Med. 2005;118:695–705. [DOI] [PubMed] [Google Scholar]


