Figure 1.
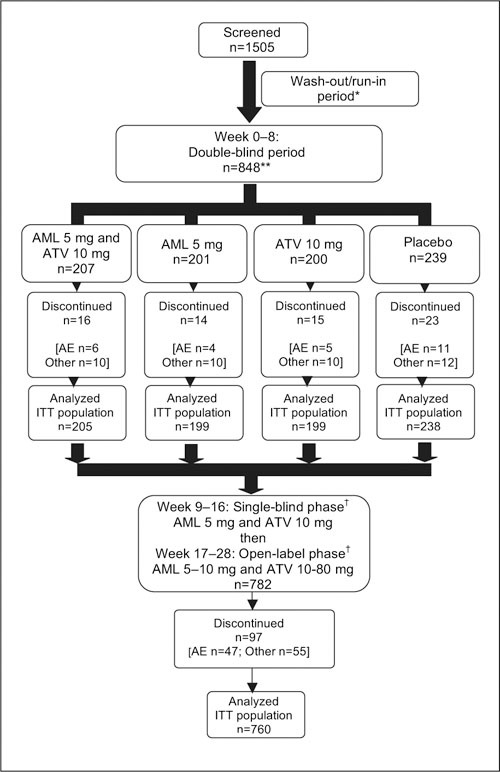
Study design and patient disposition. AE=adverse event; Other=reported lack of efficacy of drug, laboratory abnormalities, or patient defaulted (includes lack of treatment compliance); ITT=intent‐to‐treat; *duration 2–6 weeks depending on medication previously administered; **one patient randomized to treatment but did not receive drug; †amlodipine (AML) 5 mg and atorvastatin (ATV) 10 mg o.d. × 8‐week, single‐blind period, then up‐titrated to a maximum dose of AML 10 mg × ATV 80 mg, if required, during subsequent 12 weeks to achieve goals
