Extended Data Fig. 7 |. H1 promotes PRC2-mediated H3K27 trimethylation and inhibits NSD2-mediated H3K36 dimethylation in vitro.
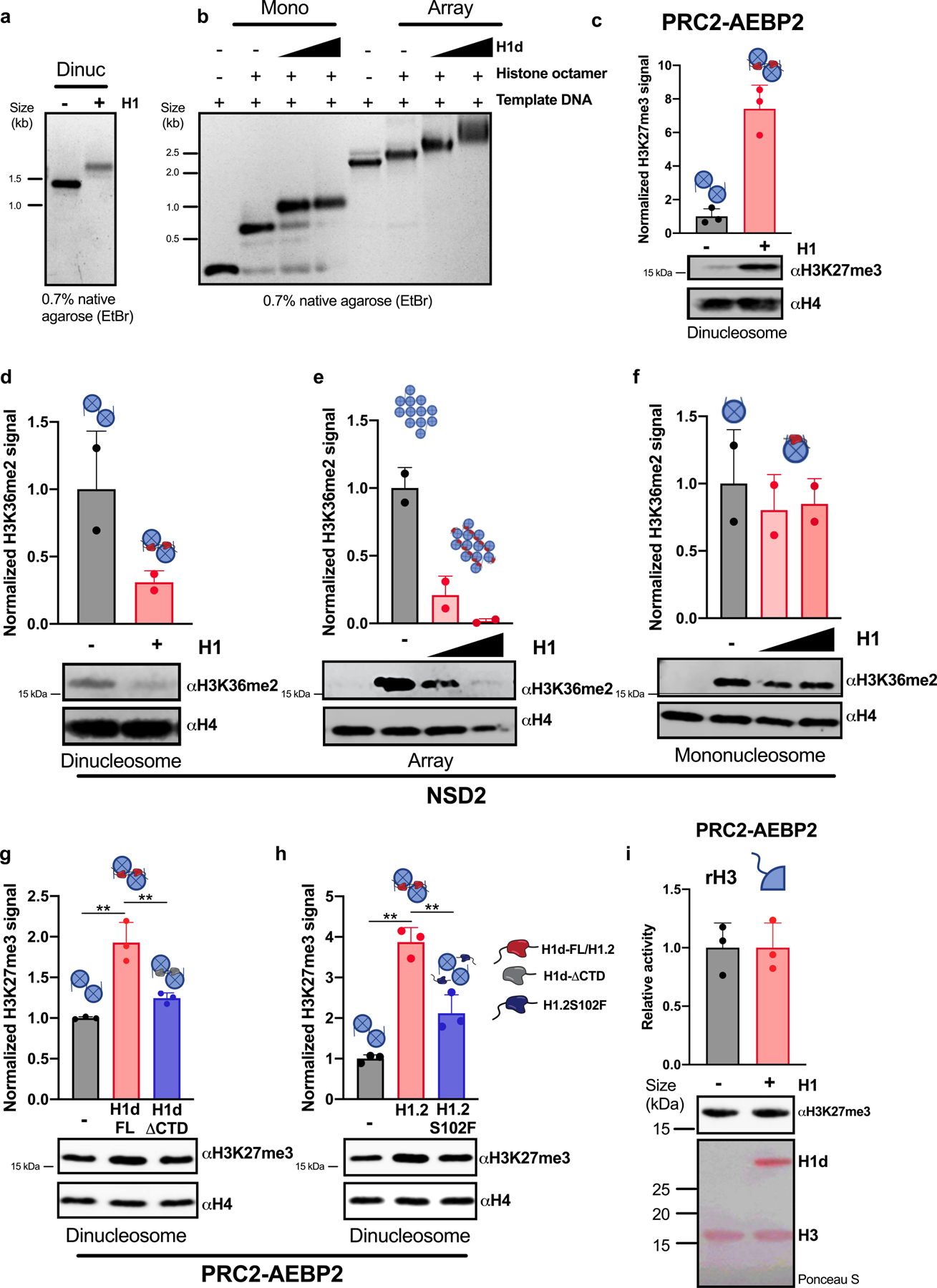
a, b, Chromatin was reconstituted in vitro using DNA templates bearing one, two or twelve repeats of the synthetic ‘601’ nucleosome positioning sequence and recombinant histone octamers either in the presence or absence of H1 proteins. Linker histone incorporation was verified by non-denaturing agarose gel electrophoresis followed by ethidium bromide staining. Representative gels are shown. Optimal H1:nucleosome ratios were determined empirically. In a, a 0.8 H1d:nucleosome ratio was used. In b, H1d:nucleosome ratios of 0.7:1 and 1.3:1 were used. c–h, In vitro histone methyltransferase (HMT) assays with reconstituted chromatin in the presence or absence of the indicated H1 proteins: (H1: M. musculus H1d; H1.2: H. sapiens H1.2; H1.2S102F; H1d-FL: H1d(1–221); H1d-ΔCTD: H1d(1–114). Chromatin was reconstituted in vitro using a DNA template bearing one (f), two (c, d, g, h) or twelve (e) repeats of the synthetic ‘601’ nucleosome positioning sequence and recombinant histone octamers either in the presence or absence of H1; optimal H1:nucleosome ratios were determined empirically. Chromatin substrates were incubated with the indicated HMT enzyme under conditions described in the Methods. HMT activity was detected by immunoblotting for the indicated histone modifications. Immunoblots of H4 are shown as a loading control. H3K27me3 and H3K36me2 signals were quantified by densitometry and normalized to the intensity of the corresponding H4 band; quantification is shown as relative activity compared to substrates in the absence of H1. i, In vitro HMT assay of histone H3 in the presence or absence of H1d. H3 was incubated with PRC2-AEBP2 complex in the presence or absence of equimolar quantities of H1d. HMT activity was detected by immunoblotting for H3K27me3. Above, quantification of activity of 3 independent HMT reactions relative to reactions lacking H1d. Middle, representative immunoblot demonstrating enzymatic activity. Below, Ponceau S stain of transferred proteins showing equal quantities of H3. Quantification of 2 (d–f) or 3 (c, g–i) independent experiments is shown above each immunoblot. Data are presented as mean values ± s.d., unpaired t-test; ns, not statistically significant; * P ≤ 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001. *For gel source data, refer to Supplementary Fig. 1.
