Abstract
This is a case report of a 63-year-old African American female with a past medical history most significant for metastatic cholangiocarcinoma that presented for evaluation of persistent shortness of breath. Initial workup was remarkable for refractory anemia, moderate schistocytes on peripheral smear and lab work suggestive of a hemolytic anemia. Due to concern for thrombotic thrombocytopenic purpura (TTP), she subsequently underwent several rounds of plasma exchange without significant improvement. Secondary to progressive renal failure, patient eventually had a renal biopsy with findings remarkable for thrombotic microangiopathy (TMA). Simultaneously, patient was also diagnosed with coronavirus disease 2019 (COVID-19) infection. After a few weeks of supportive care, she was stable for discharge. Unfortunately, she did become dialysis dependent. Prior to hospital admission, she was being treated for metastatic cholangiocarcinoma and had received chemotherapy with gemcitabine. Her last chemotherapy session was approximately 3 weeks prior to her first hospitalization. Furthermore, although her hemolytic work did suggest TMA, it was not consistent with the diagnosis of TTP. She was transferred to a tertiary care center where hemolytic labs were trended, and supportive care was maximized. In light of the current COVID-19 pandemic, it is crucial to further investigate the pathophysiology of TMA in patients with active malignancies and COVID-19 infections. To our knowledge, this is the first case of TMA in a patient with both metastatic cholangiocarcinoma and COVID-19 infection.
Keywords: COVID-19, Microangiopathic hemolytic anemia, Thrombotic microangiopathy, Cholangiocarcinoma, Gemcitabine, Complement activation
Introduction
Microangiopathic hemolytic anemia (MAHA) is defined as a non-immune hemolysis that results from intravascular red cell fragmentation observed by the production of schistocytes on peripheral smear [1]. It is characterized by abnormalities observed in the microvasculature with labs remarkable for a negative direct antiglobulin test, increased lactate dehydrogenase level, increased indirect bilirubin and low haptoglobin level. Furthermore, thrombotic microangiopathy (TMA) is used to describe syndromes characterized by MAHA along with thrombocytopenia and evidence of microvascular thrombosis. The most common TMA syndromes include thrombotic thrombocytopenic purpura (TTP), Shiga toxin-mediated hemolytic uremic syndrome (ST-HUS), drug-induced TMA (DITMA) syndromes, and complement-mediated TMA. Systemic disorders that can present with MAHA and thrombocytopenia include disseminated intravascular coagulation (DIC), systemic infections, systemic malignancies, rheumatologic diseases, and in individuals that have received either hematopoietic cell transplant or solid organ transplant (Fig. 1) [2]. The etiology of MAHA is very important and can become very complex, especially when individuals have multiple underlying conditions that can contribute to hemolysis, making treatment decisions difficult for providers. This case presentation emphasizes the need to understand the pathophysiology of viral-induced TMA in patients that have underlying malignancies, specifically the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We would like to present a complex case describing a 64-year-old lady with metastatic cholangiocarcinoma that was recently diagnosed with coronavirus disease 2019 (COVID-19), who develops TMA shortly after diagnosis, and after prolonged hospitalization and supportive care showed evidence of improvement in her thrombocytopenia and hemolysis.
Figure 1.
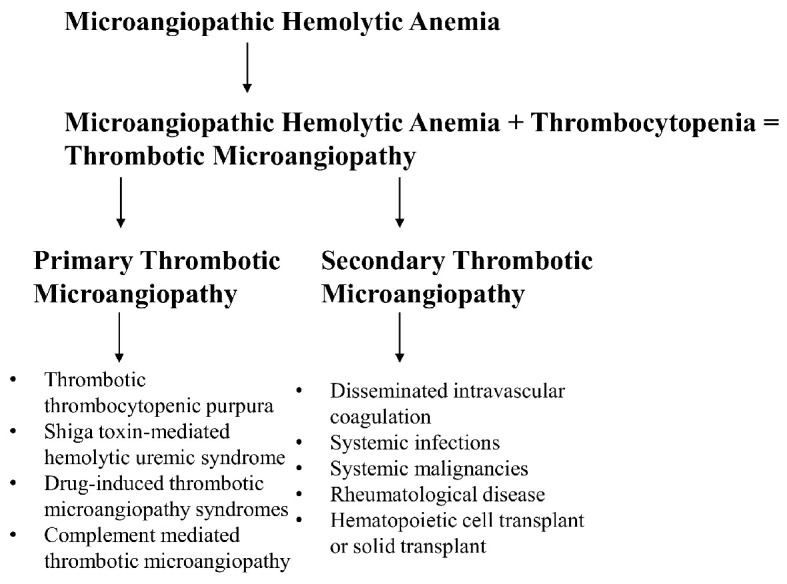
Establishing definitions.
Case Report
Investigation, diagnosis and subsequent treatment
Patient initially presented with back and right flank and became progressively jaundiced prompting emergency room evaluation at an outside facility. Computed tomography (CT) abdomen and pelvis with contrast was remarkable for a porcelain gallbladder with a large lobulated mass within the adjacent liver, resulting in intrahepatic biliary ductal dilation and subsequent endoscopic retrograde cholangiopancreatography. Fine needle aspiration confirmed the diagnosis of adenocarcinoma of gallbladder primary. Patient underwent biliary stent placement in the common bile duct and after completion of staging, started chemotherapy with cisplatin and gemcitabine. Patient was not deemed as surgical candidate. After receiving her first cycle of chemotherapy, our patient had recurrent hospitalizations and significant side effects. As a result, her oncologist started her on single agent gemcitabine. Following the third cycle of treatment, CT chest abdomen and pelvis suggested progression of disease and the decision was made to switch chemotherapy to 5-fluorouracil with leucovorin, and irinotecan (FOLFIRI).
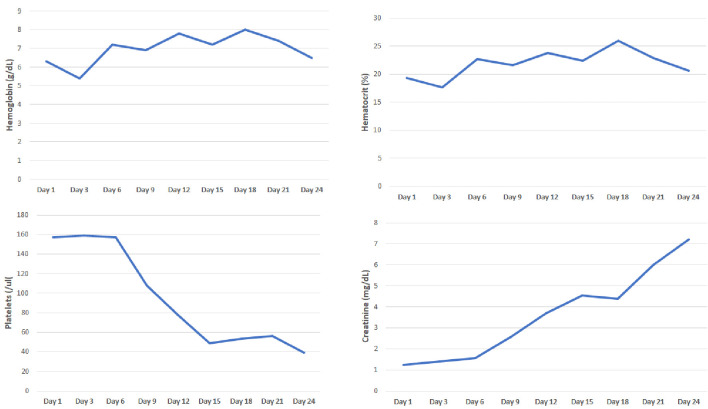
Secondary to generalized weakness and shortness of breath, patient presented to her local community hospital for evaluation. She was found to be COVID-19 positive and admitted for further evaluation. On admission, patient was found to be anemic with hemoglobin of 6.3 g/dL. She was transfused two units of packed red blood cells with an inappropriate response. Secondary to refractory anemia, hemolytic workup was initiated, which was remarkable for elevated absolute reticulocyte count, lactate dehydrogenase and moderate schistocytes on peripheral smear. Her kidney function was normal. Secondary to concern for TTP, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13) was ordered and emergent plasma exchange with steroids were arranged. Interestingly, the patient developed worsening renal failure and did not improve with plasma exchange, prompting renal biopsy. The renal biopsy was positive for TMA with predominantly acute features and additional findings of collapsing glomerulopathy with associated acute tubular injury and mild interstitial fibrosis and atrophy. Throughout her hospitalization, her lactate dehydrogenase levels remained elevated between 1,000 to 1,900 U/L (until the day of discharge), absolute reticulocyte count (in %) was 13% and ADAMTS13 level was 61.5. Patient became dialysis dependent and was discharged in stable condition (Fig. 2).
Figure 2.
Evaluation of hemoglobin, hematocrit, platelets and creatinine from first hospitalization.
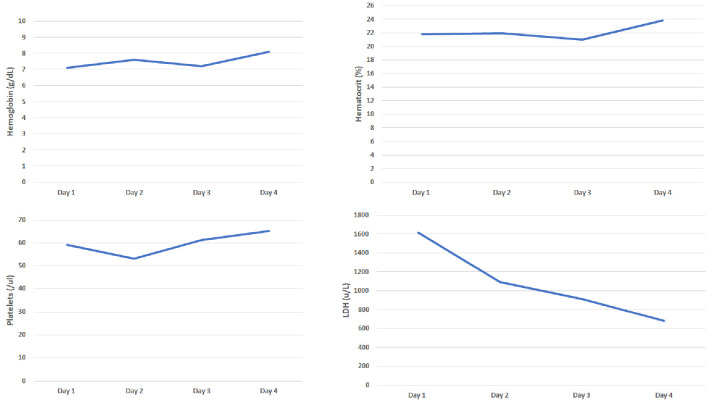
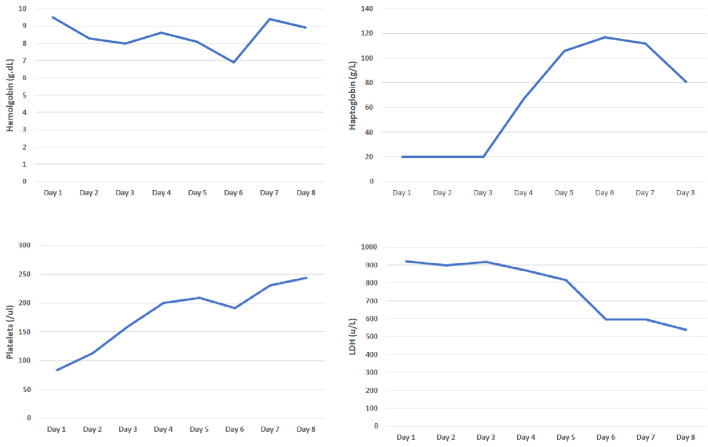
She presented to our hospital a few days later for evaluation for acute hypoxic respiratory failure. Initial lab work was remarkable for bilateral pleural effusions and elevated troponin levels which were managed by our pulmonology and cardiology service respectively. Her initial labs were remarkable for hemoglobin of 8.6 g/dL and platelet count of 96 × 109/L. Her peripheral smear was remarkable for a significant number of schistocytes prompting hemolytic workup, which was notable for increased lactate dehydrogenase levels, elevated indirect bilirubin, elevated reticulocyte count, low haptoglobin (< 30), negative direct Coombs and normal complement levels (complement 2: 2.1, complement 3: 92, complement CH50: 55). Her coagulation studies were also normal. Unfortunately, our hospital did not have records available from her first hospital visit, therefore we also were concerned for possible TTP. We subsequently ordered an ADAMTS13 and arranged for emergent plasma exchange with steroids. ADAMTS13 level at our facility was 36. At the request of the patient, she was transferred to a tertiary care center where her medical oncologist was located for further management (Fig. 3). Upon transfer to the tertiary care center, hemolytic labs were monitored closely and improved with maximizing supportive care (Fig. 4). During her hospitalization, patient developed choledocholithiasis and underwent biliary drain catheter placement.
Figure 3.
Evaluation of hemoglobin, hematocrit, platelets and lactate dehydrogenase (LDH) from our hospital.
Figure 4.
Evaluation of hemoglobin, haptoglobin, platelets, and lactate dehydrogenase (LDH) from tertiary care center.
Follow-up and outcomes
Secondary to her poor prognosis and declining functional status she was discharged with palliative care services and eventually enrolled in hospice.
Discussion
Our patient presented for evaluation at a community hospital for generalized fatigue and shortness of breath with initial labs and peripheral smear supporting the diagnosis of MAHA with associated thrombocytopenia. This diagnosis was further supported from a kidney biopsy that was remarkable for TMA with predominantly acute features and additional findings of collapsing glomerulopathy, initially concerning for acquired TTP. This case also forced us to explore cancer-associated TMA (active malignancy), drug-induced TMA (recent exposure to gemcitabine) and virus/complement-mediated TMA (active COVID-19 infection) as contributing etiologies to our patient’s clinical presentation.
Initially, we were concerned for acquired TTP secondary to the evidence of hemolysis on initial lab work, observed schistocytes on peripheral smear, and moderate to severe thrombocytopenia. The patient did not present with fevers or focal neurological deficits upon our evaluation. She did inform us that she was recently admitted for COVID-19 infection at an outside facility but was not able to provide us with much information regarding the hospitalization. We were able to obtain some records from her prior medical oncologist’s office immediately; however records from her recent hospitalization were delayed. We proceeded with further hemolytic workup as deemed appropriate.
At our facility, the ADAMTS activity was 36 (at the prior facility, it was found to be > 60 on two occasions). Although a severe deficiency of ADAMTS13 activity (typically less than 10%) is often observed in patients with acute TTP, we acknowledge that the diagnosis of TTP is not based solely on this activity level. Activity can also be reduced (< 50%) in liver disease, disseminated malignancies, and chronic metabolic and inflammatory conditions [3]. Although our patient presented with evidence of hemolysis and thrombocytopenia, our patient lacked neurological symptoms. In our literature review, we found that acquired TTP often involves the central nervous system and gastrointestinal system; the renal system is less involved [4]. Most importantly, our patient’s lack of response to plasma exchange and steroids suggested that acquired TTP was likely not the etiology of our patient’s TMA.
We considered the possibility of drug-induced TMA, which results from exposure to a drug that induces the formation of drug-dependent antibodies or causes direct tissue toxicity, resulting in the formation of platelet rich thrombi in the microvasculature. Gemcitabine is one drug that is believed to have both immune and non-immune-mediated mechanism. The mechanism does appear to be dose related in most patients over a period of weeks to months or from an unusually high individual dose. It is often observed in individuals with a cumulative dose of 20,000 mg/m2 and when the number of doses exceeds 18 [5]. Our patient’s total accumulative dose was 4,400 mg/m2 from three total doses. Although gemcitabine-related TMA has been observed in solid tumor malignancies, it is less likely that our patient’s hemolysis is related to gemcitabine secondary to very little exposure.
We also considered active malignancy as a potential cause of TMA in our patient. Cancer-associated TMA is a rare complication of metastatic solid tumors that is thought to occur when direct contact of intra-luminal tumor emboli leads to fragmentation of red blood cells. Gastric and breast cancer are the most associated cancers, but it has also been observed in cancers of the colon, gallbladder, prostate, lung, and unknown primaries [6]. Case reports of concurrent metastatic cholangiocarcinoma and TMA have also been reported; this is a very rare observation [7]. In addition to being reported in patients with disseminated malignancies, TMA has also been observed in cases of isolated invasion of the bone marrow by the tumor [3]. Consequently, tumor growth and secondary myelofibrosis in addition to abnormal angiogenesis in the bone marrow is thought to directly invade bone marrow vessels and cause the release of ultra-large von Willebrand factor (VWF) multimers. If VWF-cleaving protease activity is decreased in advanced cancer, this could potentially lead to TMA [3]. Furthermore, one case series suggests obtaining a bone marrow biopsy whenever cancer-associated TMA is considered because documentation of malignant cells in the marrow can help us differentiate this diagnosis from other types of TMA [8]. Although bone marrow is a common site of metastases, it is possible to observe cancer-free marrow too; therefore this should only be used as supportive evidence. It is treated by treatment of the underlying malignancy. Lastly, it has been cited that a variety of solid tumors can also activate the complement system. Cases have been observed in breast, ovarian and lung cancer solid tumors [9]. Many complement functions modulate tumor progression. In metastatic disease, it has been recognized that a key step in tumor progression is overcoming immune attack, which is modulated by our complement system by means of upregulation of complement regulatory proteins. Furthermore, of all complement-mediated fragments derived from complement activation, the anaphylatoxins (C3a and C5a) are known to interfere in intracellular signaling pathways and prevent effective immune and T-cell-mediated responses [10]. Could our patient’s underlying malignancy contribute to TMA?
The timing of the hemolysis occurred shortly after the patient was infected with SARS-CoV-2, prompting us to also explore the relationship between COVID-19 infection and virus-mediated TMA (Fig. 5). One proposed mechanism of COVID-19 TMA from literature is direct complement activation. The virus or the damaged host tissue can activate the complement pathways, leading to the generation of anaphylactoid molecules (C3a, and C5a) and the membrane attack complex (C5b-9). It is postulated that the formation of the membrane attack complex (MAC) causes direct endothelial injury and platelet activation leading to TMA [11]. Additionally, we know that anaphylactoid molecules interfere in tumor intracellular signaling pathways in metastatic disease [10]. Did the SARS-CoV-2 infection directly activate the complement system and exacerbate our patient’s underlying malignancy by means of complement dysregulation?
Figure 5.
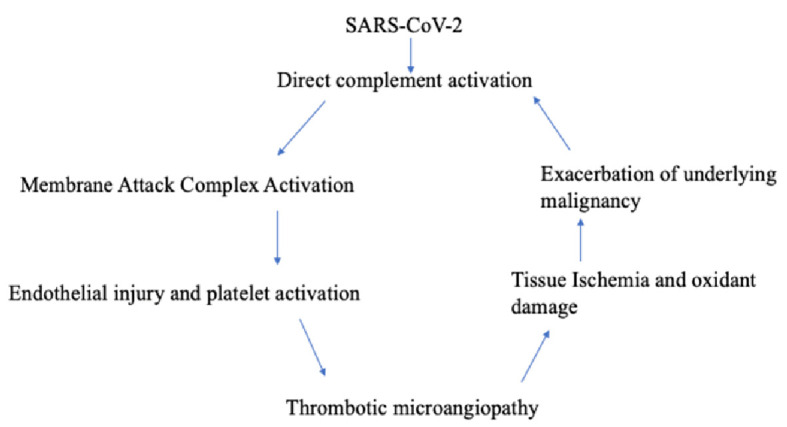
Understanding the relationship between COVID-19 and thrombotic microangiopathy in a patient with underlying malignancy. COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Management of COVID-19 complement-mediated TMA is largely supportive and is currently drawn from ongoing randomized trials and published anecdotal evidence from small uncontrolled case reports and observation studies. Complement inhibition is currently in favor for the treatment of most non-DIC thrombotic microangiopathies based on the above evidence. Unfortunately, there is little direct evidence that treatment for TMA is effective for COVID-19-associated TMA [12].
To our knowledge, this is the first case of TMA secondary to COVID-19 infection in a patient with metastatic cholangiocarcinoma. We believe that COVID-19 complement activation likely triggered a strong inflammatory cascade resulting in the production of anaphylactoid molecules and activation of the MAC complex resulting in TMA, leading to exacerbation of our patient’s underlying malignancy and additional complement dysregulation in a vicious cycle.
Acknowledgments
We thank Connor Roth, clinical oncology pharmacist for helping edit our manuscript.
Financial Disclosure
No funding was provided for this case study.
Conflict of Interest
We have no conflict of interest.
Informed Consent
Not applicable.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
References
- 1.Bhat PP, Dretler AA, Gdowski MM, et al., editors. Washington Manual of Medical Therapeutics, 35th ed. Wolters Kluwer Health; 2016. Microangiopathic Hemolytic Anemia. https://www.unboundmedicine.com/washingtonmanual/view/Washington-Manual-of-Medical-Therapeutics/602582/all/Microangiopathic_Hemolytic_Anemia. Accessed April 16, 2021. [Google Scholar]
- 2.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 3.Werner TL, Agarwal N, Carney HM, Rodgers GM. Management of cancer-associated thrombotic microangiopathy: what is the right approach? Am J Hematol. 2007;82(4):295–298. doi: 10.1002/ajh.20783. [DOI] [PubMed] [Google Scholar]
- 4.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017;1(10):590–600. doi: 10.1182/bloodadvances.2017005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zupancic M, Shah PC, Shah-Khan F. Gemcitabine-associated thrombotic thrombocytopenic purpura. Lancet Oncol. 2007;8(7):634–641. doi: 10.1016/S1470-2045(07)70203-6. [DOI] [PubMed] [Google Scholar]
- 6.Govind Babu K, Bhat GR. Cancer-associated thrombotic microangiopathy. Ecancermedicalscience. 2016;10:649. doi: 10.3332/ecancer.2016.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YT, Du HZ, Wang CY, Zhu TN, Pan BJ, Zhang L. Cholangiocarcinoma presenting with acquired thrombotic thrombocytopenic purpura confirmed by positive autoantibodies of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13. Chin Med J (Engl) 2020;133(12):1495–1496. doi: 10.1097/CM9.0000000000000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton JM, George JN. Microangiopathic hemolytic anemia and thrombocytopenia in patients with cancer. J Oncol Pract. 2016;12(6):523–530. doi: 10.1200/JOP.2016.012096. [DOI] [PubMed] [Google Scholar]
- 9.Weitz IC. Thrombotic microangiopathy in cancer. Thromb Res. 2018;164(Suppl 1):S103–S105. doi: 10.1016/j.thromres.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Ajona D, Ortiz-Espinosa S, Pio R, Lecanda F. Complement in metastasis: a comp in the camp. Front Immunol. 2019;10:669. doi: 10.3389/fimmu.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. 2020;130(8):3950–3953. doi: 10.1172/JCI140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581–589. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.