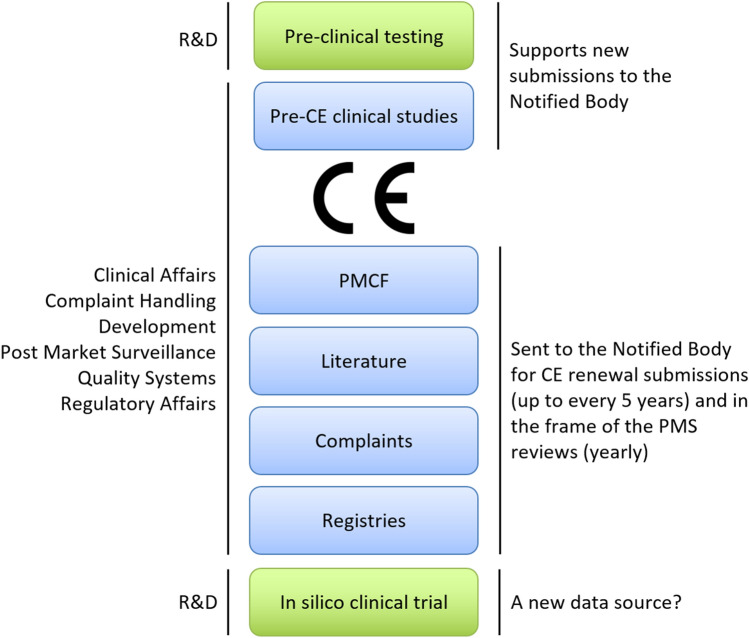
Figure 1.
Safety and performance are first established by pre-clinical testing through computer modeling and bench testing, provided by Research and Development (R&D) (green). A pre-CE clinical study is often necessary, when a medical device or its use are deemed novel. When conducting clinical evaluations for a device, four traditional sources can be included (blue): 1. Clinical studies (pre-CE clinical studies or post-market clinical follow-up studies—PMCF), 2. Literature, 3. Complaints, and 4. Registries. After receipt of the CE mark by the regulator, the product can be launched to market provided a clear plan for collecting clinical data is available. In parallel with market launch, PMCF studies are established to proactively collect data. The scientific literature, in particular peer-reviewed publications, are screened to ensure that the device offers the intended benefits and to detect potential adverse events. All implant-related complaints directly received by the manufacturer or reported in internal clinical studies or published literature studies are analyzed in post-market surveillance (PMS). Registries are also a great source of long-term data as they follow large cohorts and cover several implant designs.26 Despite these potential sources, data may be lacking for rare implants, demographics, and indications. ISCT may fill these gaps, and may become the fifth data source.