Figure 1.
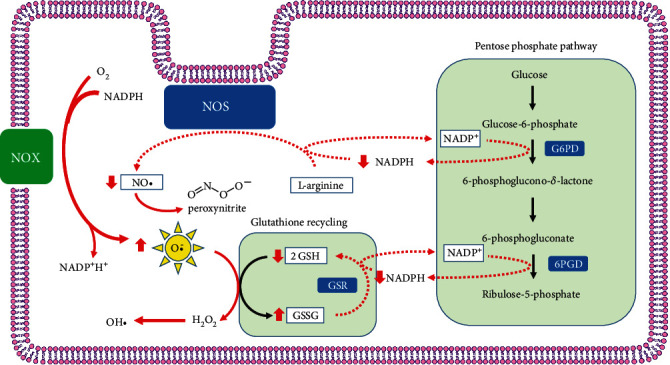
Pentose phosphate pathway (PPP) and glucose 6-phosphate dehydrogenase (G6PD) in nucleated cells. The NADPH provides reducing equivalents for antioxidant defense and reductive biosynthesis and NADPH oxidase for generation of superoxide anions. The G6PD is required to maintain a normal NADPH/NADP ratio which in turn regulates the glutathione (γ-L-glutamyl-L-cysteinylglycine, GSH) biosynthesis. The GSH is a sulfhydryl-containing compound present in all mammalian cells. The redox-active thiol group in GSH is essential in the regulation of disulfide bonds of proteins and to detoxify oxidant compounds. The function of GSH as an antioxidant is efficient when the free thiol is maintained. This is accomplished by the reaction catalyzed by the NADPH-dependent glutathione-disulfide reductase (GSR) that reduces glutathione disulfide (GSSG) into the form with a free thiol (GSH). In G6PD deficiency, the decreased supply of NADPH limits the GSH regeneration and in turn the disposal of oxidants. The NADPH produced in the PPP is also a substrate for nitric oxide synthase (NOS) for the release of nitric oxide (NO) and for NADPH oxidase for the release of superoxide anion. In G6PD deficiency, NO depletion leads to the decreased neutralization of superoxide anion and other free radicals.
