Abstract
Exosomes are nanometer-sized vesicles that contain various types of biologically active components, including proteins, nucleic acids, carbohydrates, and lipids, which vary with the type and physiological state of the cell. In recent years, several studies have showed that exosomes can provide new non-invasive diagnostic and prognostic biomarkers in patients affected by cancers, including bladder cancer (BC), and the lipid bilayer membrane structure makes exosomes as promising delivery vehicles for therapeutic applications. Exosomes have the characteristics of high abundance, high stability, tissue specificity, and wide distribution in body fluids, and are secreted as various types by cells in different states, thereby possessing great potential as biomarkers for BC. Herein, we briefly summarize the functions and roles of exosomes in the occurrence and development of BC and the current progress of research on exosomes in BC, while focusing on potential clinical applications of the diagnosis, treatment, and prognosis of BC.
Keywords: Exosome, Bladder cancer, Function, Clinical application, Biomarker
1 Introduction
Bladder cancer (BC) is the second most malignant tumor of the urinary tract, and the fifth most common cancer worldwide (Liang et al., 2017; Christensen et al., 2019). It has a significant incidence and mortality rate with annually 80 470 new cases diagnosed and 17 670 deaths recorded worldwide (Li et al., 2019; Siegel et al., 2019). The cancer in approximately 75% of newly diagnosed BC patients is of the non-muscle invasive type, of which about 70% of cases are confined to the mucosa, 20% involve the submucosa, and 10% are carcinoma in situ. Nevertheless, 1%–45% of non-muscle invasive patients will still develop muscle invasive BC (Mearini et al., 2017). In addition, BC has the characteristics of high relapse rate, rapid progress, and poor prognosis (Wang et al., 2018). The risks of recurrence and malignant progression of BC were found to increase with tumor stage, degree of malignancy, pathological changes, and the presence of carcinoma in situ (Mearini et al., 2017). Early diagnosis and monitoring is the key to improve the therapeutic effect in BC. Current BC diagnosis and monitoring techniques usually involve a combination of cystoscopy, histological evaluation, and urine cytology (Miyamoto et al., 2018; Wang et al., 2018). These methods, however, are uncomfortable, invasive and costly, and have low patient acceptance. Also, due to the lack of sensitivity and specificity, the early diagnostic application value of BC is limited (Li et al., 2019; Zhang et al., 2019). For example, urine cytology has a weakness of low sensitivity (about 30%–92%), which is even lower (only 30%–40%) for low-grade tumors. The other most common diagnostic method is cystoscopy, which is invasive, painful, and potentially infectious (Poli et al., 2015, 2017). All of these deficiencies of current techniques highlight the urgent need to develop novel non-invasive methods and more sensitive biomarkers for BC diagnosis and treatment.
The concept of "liquid biopsy" was proposed to characterize the utility of circulating cancer cells as non-invasive cancer biomarker candidates (Pantel and Alix-Panabières, 2010). The significance of this novel approach lies in the fact that it was also found to involve the analysis of circulating subcellular structures, such as exosomes, cell-free DNA, and other substances (de Palma et al., 2019). These exosomes, as extracellular vesicles that can be secreted by almost all cells, have attracted increased attention in tumor diagnosis and treatment in recent years.
Exosomes are nanometer-sized vesicles sized 30–150 nm that contain a variety of active components, including proteins, nucleic acids, carbohydrates, and lipids, which vary with the type and the physiological state of the cell that secretes the exosome (Chen et al., 2020). Exosomes have lipid bilayer membranes to protect these active components from RNases and proteases (Pegtel and Gould, 2019). It has been reported that the proteins and lipids on the surface of exosomes may vary according to the type of cell of origin, which makes it possible to further isolate and recognize bladder cell-derived exosomes (Street et al., 2014). More importantly, exosomes have been shown to be involved in cellular communication and cancer progression by interacting with target cells and delivering exosomal cargo into target cells (Franzen et al., 2016). The possibility to isolate exosomes from body fluids, such as blood and urine, provides a new non-invasive biomarker diagnostic tool in cancer patients, and the lipid bilayer membrane makes exosomes as promising delivery vehicles for therapeutic applications (Franzen et al., 2016). In addition, exosomes carry certain substances such as specific microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and proteins, which increase the diagnostic sensitivity and specificity of tumor markers, including those of BC.
2 Role of exosomes in the occurrence and progression of BC
In recent years, exosomes have attracted much attention as important messengers of cancer-related intercellular communication. They carry proteins, lipids, nucleic acids, and other biomolecules, such as surface molecules that can induce signal transduction through a receptor‒ligand interaction. In addition, exosomes can be internalized through endocytosis and/or phagocytosis, or even fuse with the target cell membrane to transport their contents into the cytoplasm ( Lin et al., 2020). Exosomes play a key role in the transmission of proteomics and genetic information to target cells. Evidence has been accumulating to highlight that exosomes from donor cells can alter the state of recipient cells and may play a crucial role in the process of tumor formation and development (el Andaloussi et al., 2013; Lin et al., 2020).
Several reports have shown that exosomes are highly important in tumorigenesis, progression, and metastasis for their role of transferring carcinogenic molecules (Zhan et al., 2018; Yoshida et al., 2019). The prognosis of BC patients with clinical lymph node metastasis is extremely poor. Exosomal lncRNA LNMAT2 secreted by BC cells was internalized by human lymphatic endothelial cells, triggering the epigenetic upregulation of Prox1 expression through hnRNPA2B1 recruitment and the increase of H3K4 trimethylation in the Prox1 promoter, which eventually leads to lymphangiogenesis and lymphatic metastasis in BC cells (Chen et al., 2020). Progressive and metastatic invasive BC generally has a poor prognosis. Exosomal cysteine-rich receptor-like kinase (CRK) receptors secreted by BC cells increase epidermal growth factor receptor 2/3 (ErbB2/3) expression in other BC cells. These kinases/adaptors transfer from host BC cells to recipient cells through exons, inducing vascular leakage and proliferation, and leading to distant metastasis (Yoshida et al., 2019). Intratumoral hypoxia is a tumor microenvironment that is not conducive to the rapid expansion of tumors. However, cancer cells can reshape the surrounding microenvironment, maintain survival and growth, and subsequently promote invasion and metastasis. Hypoxic BC cells were demonstrated to secrete lncRNA urothelial cancer associated 1 (UCA1)-enriched exosomes to reshape the tumor microenvironment and promote tumor growth and progress (Xue et al., 2017). Exosomal lncRNA UCA1 in human serum can potentially be used as a diagnostic biomarker for BC. As a new type of non-coding RNAs, circRNAs have received a lot of attention in the pathogenesis of BC and other tumors in recent years. For example, Chenet al. (2018) found that circPRMT5 expression was increased in serum and urine exosomes of BC patients, and was significantly associated with BC metastasis.
Exosomes secreted by cancer cells can also affect normal cell function, thereby promoting the occurrence of BC. Wuet al. (2019) showed that long-term exposure to cancer-derived exosomes induces the malignant transformation of urothelial cells. These transformed cells not only have a range of carcinogenic properties, such as increased genomic instability and invasiveness or loss of cell‒cell contact inhibition, but also show changes in morphology and cell structure, including cytoplasmic enlargement, small volume fusion of mitochondria, and others (Wu et al., 2019). Franzen et al. (2015) reported that the expression of mesenchymal markers (including α-smooth muscle actin (α-SMA), S100A4, and Snail) was increased and the expression of epithelial markers (such as E-cadherin and β-catenin) was decreased in urothelial cells treated by BC-derived exosomes, and further showed that BC-derived exosomes increased the migration and invasion characteristics of urothelial cells.
Exosomes also can inhibit or delay tumorigenesis and progression by transporting tumor suppressor molecules. MiRNAs can be packaged into exosomes to mediate cell-to-cell communication, affecting cancer cell proliferation, metastasis, and apoptosis. Li et al. (2020) demonstrated that exosomal miR-375-3p from BC cells inhibits cell proliferation and metastasis, and promotes cell apoptosis both in in vivo and in vitro models as a suppressor of BC, which can be used as a potential therapeutic approach for BC. The malignant proliferation and migration ability of BC cells plays a crucial role in the occurrence and development of BC. It was reported that miR-133b plays an important role in inhibiting the proliferation and promoting the apoptosis of BC cells by upregulating dual-specificity phosphatase 1 (DUSP1) expression, and this finding is expected to provide a new direction for BC treatment (Cai et al., 2020).
Exosomes secreted by other cell types have also been found to play a key role in BC occurrence and progression. Exosomes secreted by bone marrow mesenchymal stem cells, for example, significantly contribute to the occurrence and development of BC. Cai et al. (2019) found that exosomal miR-9-3p derived from bone marrow mesenchymal stem cells inhibits the progression of BC by downregulating endothelial cell-specific molecule 1 (ESM1), which may point to a new potential target for the treatment of BC. Fibroblast cells, as tumor microenvironment cells that respond to cancer cells, are activated and exhibit the characteristics of myofibroblasts, which is favorable for aggressive growth and metastasis. Ringuette Goulet et al. (2018) demonstrated that BC cells trigger the differentiation of fibroblasts to cancer-associated fibroblasts by exosome-mediated transforming growth factor-β (TGF-β) transfer and the activation of Sma- and Mad-related protein (SMAD) pathway, which plays a key role in the progression of BC.
Exosomes play a highly important role in the occurrence and development of BC, as demonstrated by various studies (Table 1). An increasing number of studies are focused on the relationship between exosomes and BC, aiming to determine specific mechanisms of action (Beckham et al., 2014; Berrondo et al., 2016; Huang et al., 2020; Yin et al., 2020); relevant results will be beneficial for BC diagnosis, treatment, and prognostic judgment.
Table 1.
Role of exosomes in the occurrence and progression of BC
| Sample | Role in BC | Functional component | Reference |
|---|---|---|---|
| BC cell exosomes | Induce lymphangiogenesis and lymphatic metastasis | LncRNA LNMAT2 | Chen et al., 2020 |
| BC cell exosomes | Induce vascular growth in premetastatic niches and promote metastasis | ErbB2 and CRK | Yoshida et al., 2019 |
| BC cell exosomes; serum exosomes from BC patients | Remodel tumor microenvironment; promote tumor growth and development | LncRNA UCA1 | Xue et al., 2017 |
| Serum and urine exosomes from BC patients | Be correlated with tumor metastasis | CircPRMT5 | Chen et al., 2018 |
| Urine exosomes from BC patients | Promote cancer progression | EDIL-3 | Beckham et al., 2014 |
| BC cell exosomes | Induce malignant transformation of SV-HUC cells | Wu et al., 2019 | |
| BC cell exosomes; urine exosomes from BC patients | Induce EMT and promote invasion in urothelial cells | Franzen et al., 2015 | |
| BC cell exosomes | Inhibit proliferation and metastasis; promote apoptosis | miR-375-3p | Li et al., 2020 |
| BC cell exosomes | Inhibit proliferation and promote apoptosis | miR-133b | Cai et al., 2020 |
| Bone marrow-derived mesenchymal stem cell exosomes | Inhibit BC cell viability, migration, and invasion; induce apoptosis | miR-9-3p | Cai et al., 2019 |
| BC cell exosomes | Trigger the differentiation of fibroblasts to cancer-associated fibroblasts | TGF-β | Ringuette Gouletet al., 2018 |
BC: bladder cancer; LncRNA: long non-coding RNA; ErbB2: epidermal growth factor receptor 2; CRK: cysteine-rich receptor-like kinase; UCA1: urothelial cancer associated 1; EDIL-3: endothelial locus 3; SV-HUC: SV-40 immortalized human ureteral epithelial cells; EMT: epithelial-mesenchymal transition; TGF-β: transforming growth factor-β.
3 Application of exosomes in the diagnosis and prognosis of BC
As discussed above, exosomes contain essential functional components, including proteins, lipids, nucleic acids, and transcription factors. These functional biomolecules are not randomly packaged in exosomes, but depend on characteristic motifs determining the carried active components under different conditions or in different cell types (Zhan et al., 2018; Chen et al., 2020). Studies have confirmed that exosomes secreted by BC cells can be found in the blood and the urine, and functional components, such as proteins and nucleic acids, are stably present in these exosomes (Zhan et al., 2018). Therefore, the analysis of exosomes and their components in body fluids is expected to provide valuable clues for BC diagnosis.
Exosomes play a role in BC progression by encapsulating lncRNAs to mediate extracellular communication, and thus can be used as potential non-invasive biomarkers for BC detection. The diagnostic accuracy of lncRNA phosphatase and tensin homolog pseudogene 1 (PTENP1) was evaluated by the area under the curve (AUC) of the receiver operating characteristic curve. Specifically, Zheng et al. (2018) found that exosomal lncRNA PTENP1 could relatively correctly distinguish BC patients from healthy controls with an AUC value of 0.743; it was further discovered in the study that exosomes secreted by normal cells can transfer lncRNA PTENP1 to BC cells, which inhibit the invasion and migration capabilities, increase the apoptosis, and reduce the malignant biological behavior of BC cells in vitro. Zhan et al. (2018) established a group composed of three different expressed lncRNAs (metastasis-associated lung denocarcinoma transcript 1 (MALAT1), prostate cancer-associated non-coding RNA transcript-1 (PCAT-1), and SPRY4-intronic transcript 1 (SPRY4-IT1)) in urine for BC diagnosis, with an AUC value of 0.854, which was significantly higher than that of urine cytology, and in addition these results also demonstrated that exosomal lncRNA PCAT-1 may be an independent prognostic indicator of BC. Zhang et al. (2019) identified a panel of three lncRNAs (PCAT-1, up-regulated in bladder cancer 1 (UBC1), and small nucleolar RNA host gene 16 (SNHG16)) providing high diagnostic accuracy for BC, with the AUC of training set and verification set at 0.857 and 0.826, respectively, which were significantly higher than those of urine cytology. The corresponding AUC values of Ta, T1, and T2‒T4 in the panel for BC patients were 0.760, 0.827, and 0.878, respectively. It was also found that recurrence free survival was significantly lower in non-muscle invasive BC patients with higher lncRNA UBC1 expression (Zhang et al., 2019). The clinical significance of urine exosomal antisense non-coding RNA in the INK4 locus (ANRIL) and PCAT-1 as biomarkers was evaluated in BC patients classified as T1 or T2 by Abbastabar et al. (2020). Compared with normal subjects, the expression of ANRIL and PCAT-1 in BC patient urine exosomes was significantly higher, and the diagnostic efficacies of ANRIL and PCAT-1 detected by AUC were 0.7229 and 0.7292, respectively. The above results suggest that body fluid exosomes may be used as potential important biomarkers for the diagnosis and prognosis of BC.
Exosomes are regarded as emerging tools for cancer diagnosis; more and more research is conducted on the application of exosomes in the diagnosis, prognosis, and risk assessment of BC (Yazarlou et al., 2018a, 2018b; Elsharkawi et al., 2019; Poli et al., 2020). These studies elaborate on cancer-testis antigens, the secretion of exosomes at different stages of BC, and the active molecules in exosomes such as nucleic acids and proteins, all providing new strategies for BC diagnosis and treatment.
4 Current challenges and future prospects
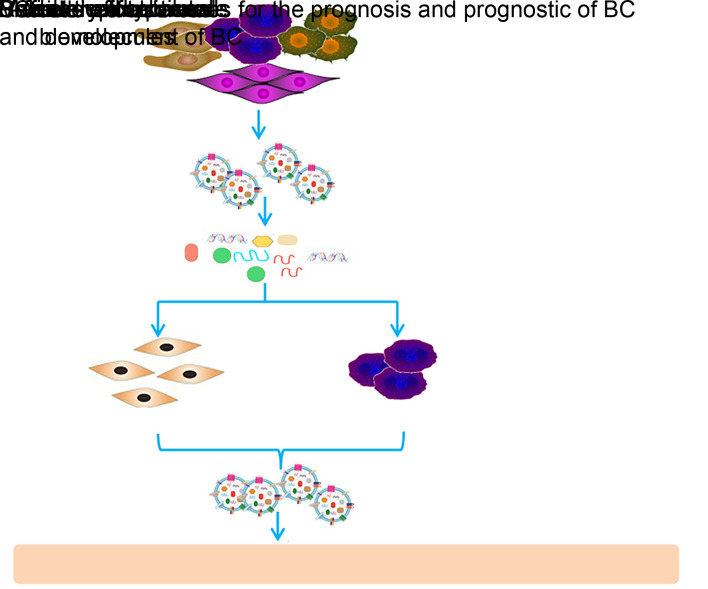
Exosomes provide the possibility of non-invasive observation of bladder cells, enhancing the potential to establish the occurrence and development stage of BC (Fig. 1). At present, however, the vast majority of exosomal biomarkers are only used as research tools. In order to reach the level of their successful clinical application, it is necessary to utilize the experience of establishing mature biomarkers, focus on supporting decision making in the nursing of BC patients, and eliminate the obstacles of clinical application (Street et al., 2014).
Fig. 1. Important roles of exosomes in the occurrence, progression, and diagnosis of bladder cancer (BC).
Despite the prospect of exosomes being utilized in fields of BC diagnosis, prognosis, and risk assessment research, many unresolved questions remain in experimental and clinical research. Firstly, there is a shortage of efficient and standardized exosome extraction and identification methods. Secondly, the packaging mechanism of active molecules carried by BC-related exosomes is still unclear. Thirdly, the number of functional components carried in exosomes with known biological function and mechanism of action is limited in BC. Furthermore, the conditions under which exosomes play a cancer‒promoting effect or a cancer-suppressing effect are yet to be investigated. It is also unclear whether exosomes can influence the BC cell microenvironment and thus play a role in BC progression.
Nevertheless, the successful clinical application of exosomes as targets or drug delivery vehicles in BC requires more detailed and complete experimental data, such as that on safety, targeting ability, efficacy, and many other aspects; a systematic assessment including the cost, accuracy, and repeatability of exosomal biomarker analysis in a large number of BC samples, is also lacking.
5 Conclusions
Exosomes contain various types of biologically active components, including proteins, nucleic acids, carbohydrates, and lipids, and have been regarded as emerging tools for cancer diagnosis. The analysis of exosomes and their components from body fluids has a great potential to provide valuable clues for the diagnosis and prognosis of BC. In the current stage, however, knowledge on exosomes regarding the precise mechanisms of secretion, selective transport of functional components, and biological function in BC, is still limited. Nevertheless, we consider that these constraints will eventually be resolved, and novel diagnosis and treatment strategies based on exosomes carrying active molecules will be established to provide efficient tools for BC clinical diagnosis, prognosis, and treatment tasks.
Acknowledgments
The present work was supported by the National Natural Science Foundation of China (No. 8157100782), the Natural Science Research in Universities of Anhui Province (No. KJ2018A0209), the Foundation for Excellent Young Teachers of Jiangsu University, Zhenjiang Social Development Guidance Project (No. FZ2019038), the Research and Practice Innovation Program for Graduate Students of Jiangsu Province (No. KYCX20_3089), and the Experimental Animal Center of Jiangsu University, China.
Author contributions
Hao GENG, Qingchen ZHOU, and Zhaofeng LIANG designed research and wrote the paper. Wenhao GUO, Ling LU, Liangkuan BI, Yi WANG, and Jie MIN participated in data collection and analysis. Dexin YU and Zhaofeng LIANG participated in the writing and revisions. All authors have read and approved the final manuscript.
Compliance with ethics guidelines
Hao GENG, Qingchen ZHOU, Wenhao GUO, Ling LU, Liangkuan BI, Yi WANG, Jie MIN, Dexin YU, and Zhaofeng LIANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abbastabar M, Sarfi M, Golestani A, et al. , 2020. Tumor-derived urinary exosomal long non-coding RNAs as diagnostic biomarkers for bladder cancer. EXCLI J, 19: 301-310. 10.17179/excli2019-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham CJ, Olsen J, Yin PN, et al. , 2014. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol, 192(2): 583-592. 10.1016/j.juro.2014.02.035 [DOI] [PubMed] [Google Scholar]
- Berrondo C, Flax J, Kucherov V, et al. , 2016. Expression of the long non-coding RNA hotair correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE, 11(1): e0147236. 10.1371/journal.pone.0147236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HZ, Yang XJ, Gao Y, et al. , 2019. Exosomal microRNA-9-3p secreted from BMSCs downregulates ESM1 to suppress the development of bladder cancer. Mol Ther Nucleic Acids, 18: 787-800. 10.1016/j.omtn.2019.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XX, Qu LL, Yang J, et al. , 2020. Exosome-transmitted microRNA-133b inhibited bladder cancer proliferation by upregulating dual-specificity protein phosphatase 1. Cancer Med, 9(16): 6009-6019. 10.1002/cam4.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Luo YM, He W, et al. , 2020. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest, 130(1): 404-421. 10.1172/JCI130892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen RX, Wei WS, et al. , 2018. PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin Cancer Res, 24(24): 6319-6330. 10.1158/1078-0432.CCR-18-1270 [DOI] [PubMed] [Google Scholar]
- Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. , 2019. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol, 37(18): 1547-1557. 10.1200/JCO.18.02052 [DOI] [PubMed] [Google Scholar]
- de Palma G, di Lorenzo VF, Krol S, et al. , 2019. Urinary exosomal shuttle RNA: promising cancer diagnosis biomarkers of lower urinary tract. Int J Biol Markers, 34(2): 101-107. 10.1177/1724600819827023 [DOI] [PubMed] [Google Scholar]
- el Andaloussi S, Mäger I, Breakefield XO, et al. , 2013. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov, 12(5): 347-357. 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- Elsharkawi F, Elsabah M, Shabayek M, et al. , 2019. Urine and serum exosomes as novel biomarkers in detection of bladder cancer. Asian Pac J Cancer Prev, 20(7): 2219-2224. 10.31557/APJCP.2019.20.7.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen CA, Blackwell RH, Todorovic V, et al. , 2015. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis, 4(8): e163. 10.1038/oncsis.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen CA, Blackwell RH, Foreman KE, et al. , 2016. Urinary exosomes: the potential for biomarker utility, intercellular signaling and therapeutics in urological malignancy. J Urol, 195(5): 1331-1339. 10.1016/j.juro.2015.08.115 [DOI] [PubMed] [Google Scholar]
- Huang CS, Ho JY, Chiang JH, et al. , 2020. Exosome-derived LINC00960 and LINC02470 promote the epithelial-mesenchymal transition and aggressiveness of bladder cancer cells. Cells, 9(6): 1419. 10.3390/cells9061419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang HL, Peng HR, et al. , 2019. MicroRNAs: key players in bladder cancer. Mol Diagn Ther, 23(5): 579-601. 10.1007/s40291-019-00410-4 [DOI] [PubMed] [Google Scholar]
- Li Q, Huyan T, Cai SN, et al. , 2020. The role of exosomal miR-375-3p: a potential suppressor in bladder cancer via the Wnt/β-catenin pathway. FASEB J, 34(9): 12177-12196. 10.1096/fj.202000347R [DOI] [PubMed] [Google Scholar]
- Liang ZF, Lu L, Mao JH, et al. , 2017. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Discov, 8(10): e3066. 10.1038/cddis.2017.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Yin HB, Li XY, et al. , 2020. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int J Oncol, 56(1): 151-164. 10.3892/ijo.2019.4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mearini E, Poli G, Cochetti G, et al. , 2017. Expression of urinary miRNAs targeting NLRs inflammasomes in bladder cancer. Onco Targets Ther, 10: 2665-2673. 10.2147/OTT.S132680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Mouw KW, Feng FY, et al. , 2018. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol, 19(12): e683-e695. 10.1016/S1470-2045(18)30693-4 [DOI] [PubMed] [Google Scholar]
- Pantel K, Alix-Panabières C, 2010. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med, 16(9): 398-406. 10.1016/j.molmed.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Gould SJ, 2019. Exosomes. Annu Rev Biochem, 88: 487-514. 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- Poli G, Brancorsini S, Cochetti G, et al. , 2015. Expression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNA. Urol Oncol Semin Orig Invest, 33(12): 505.e1-505.e7. 10.1016/j.urolonc.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Poli G, Cochetti G, Boni A, et al. , 2017. Characterization of inflammasome-related genes in urine sediments of patients receiving intravesical BCG therapy. Urol Oncol Semin Orig Invest, 35(12): 674.e19-674.e24. 10.1016/j.urolonc.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Poli G, Egidi MG, Cochetti G, et al. , 2020. Relationship between cellular and exosomal miRNAs targeting NOD-like receptors in bladder cancer: preliminary results. Minerva Urol Nefrol, 72(2): 207-213. 10.23736/S0393-2249.19.03297-1 [DOI] [PubMed] [Google Scholar]
- Ringuette Goulet C, Bernard G, Tremblay S, et al. , 2018. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Mol Cancer Res, 16(7): 1196-1204. 10.1158/1541-7786.MCR-17-0784 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2019. Cancer statistics, 2019. CA Cancer J Clin, 69(1): 7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Street JM, Yuen PST, Star RA, 2014. Bioactive exosomes: possibilities for diagnosis and management of bladder cancer. J Urol, 192(2): 297-298. 10.1016/j.juro.2014.05.050 [DOI] [PubMed] [Google Scholar]
- Wang JS, Yang K, Yuan WX, et al. , 2018. Determination of serum exosomal H19 as a noninvasive biomarker for bladder cancer diagnosis and prognosis. Med Sci Monit, 24: 9307-9316. 10.12659/MSM.912018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Silvers CR, Messing EM, et al. , 2019. Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells. J Biol Chem, 294(9): 3207-3218. 10.1074/jbc.RA118.006682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Chen W, Xiang A, et al. , 2017. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer, 16: 143. 10.1186/s12943-017-0714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazarlou F, Mowla SJ, Kholghi Oskooei V, et al. , 2018a. Urine exosome gene expression of cancer-testis antigens for prediction of bladder carcinoma. Cancer Manage Res, 10: 5373-5381. 10.2147/CMAR.S180389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazarlou F, Modarressi MH, Mowla SJ, et al. , 2018b. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manage Res, 10: 6357-6365. 10.2147/CMAR.S186108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XB, Zheng XP, Liu M, et al. , 2020. Exosomal miR-663b targets Ets2-repressor factor to promote proliferation and the epithelial-mesenchymal transition of bladder cancer cells. Cell Biol Int, 44(4): 958-965. 10.1002/cbin.11292 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Tsuda M, Matsumoto R, et al. , 2019. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci, 110(7): 2119-2132. 10.1111/cas.14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Du LT, Wang LS, et al. , 2018. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer, 17: 142. 10.1186/s12943-018-0893-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Du LT, Wang LS, et al. , 2019. Evaluation of serum exosomal lncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med, 23(2): 1396-1405. 10.1111/jcmm.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Du ML, Wang XW, et al. , 2018. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer, 17(1): 143. 10.1186/s12943-018-0880-3 [DOI] [PMC free article] [PubMed] [Google Scholar]