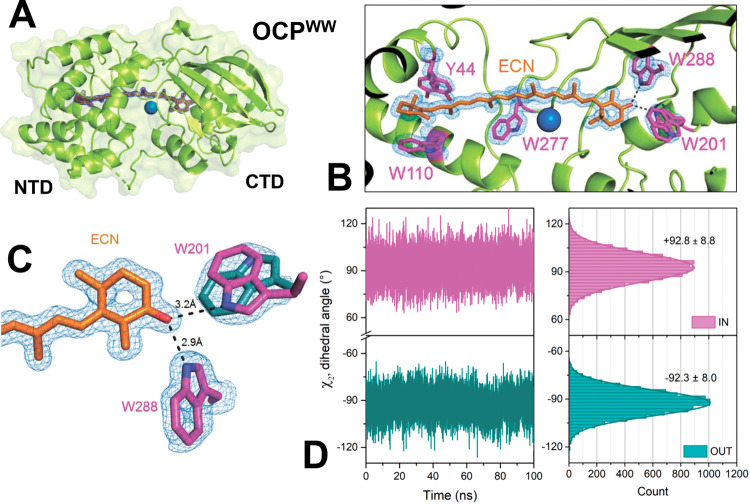
Fig. 2. The most complete, representative crystal structure of the Y201W variant of OCP (OCPWW), PDB ID 6T6O.
A Overall structure of ECN-binding OCPWW shown as a cartoon backbone and a semi-transparent surface. A chloride ion is shown as a blue sphere. B Close-up view on the carotenoid-binding pocket of OCPWW. Crucial amino acids with hydrophobic side chains are shown in purple. Note the alternative positions of Trp-201 and Tyr-44. The other structures determined at different pH values (PDB IDs 6T6K, 6T6M) also reveal two alternative conformations for these amino acids. C Organization of hydrogen bonds between the keto oxygen of ECN and Trp-288/Trp-201. Panels A–C show 2FO-FC electron density maps contoured at 3σ (A) or 1σ (B, C). D Values of the dihedral angle (χ2) between the peptide backbone and the indole ring of Trp-201 as revealed by MD simulations of OCPWW with different initial conformation of Trp-201 (as shown in panel C: IN – purple, OUT – cyan).