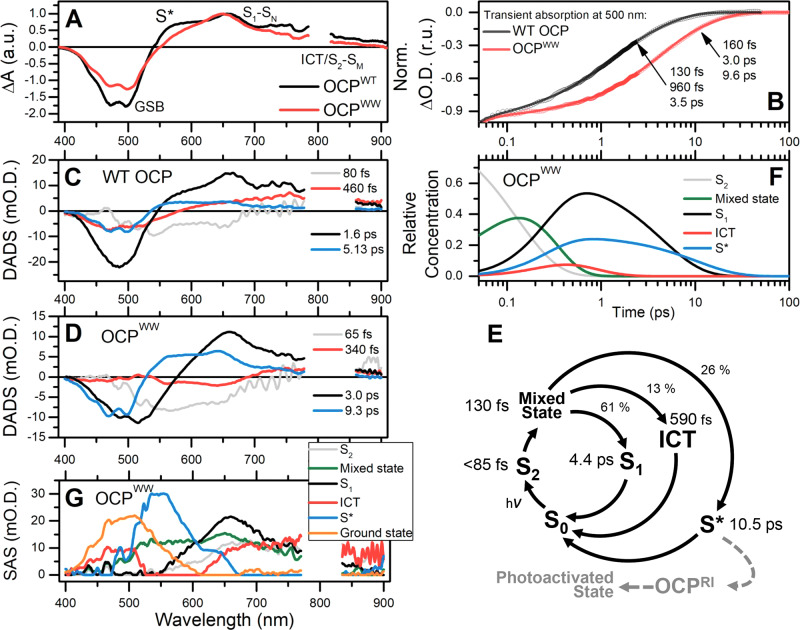
Fig. 3. Excited state dynamics and photochemistry of the ketocarotenoid in OCP as revealed by transient absorption spectroscopy.
A Transient absorption spectra of WT OCP (black) and OCPWW (red). Spectra were recorded 1 ps after excitation with a 30-fs pulse at 520 nm for WT OCP and OCPWW. Spectra were normalized to the maximum of the ESA bands. B Time courses of transient absorption of WT OCP (black) and OCPWW (red) measured at 500 nm probe wavelength. Numbers indicate the characteristic lifetimes of the corresponding states. Note the logarithmic timescale. C, D Decay-associated difference spectra (DADS) obtained from global fitting of the transient absorption spectra of WT OCP and OCPWW, respectively. E The model of transitions between the states of ECN in OCP after photoexcitation. Numbers indicate characteristic lifetimes of the corresponding states. OCPRI represents a long-lived “red intermediate” OCP state, in which the protein structure is compact, while the conformation of the carotenoid is (quasi-)stabilized in a state with an increased conjugation length due to breakage of hydrogen bonds to the amino acid residues in the CTD. The following dashed arrow indicates possible slow transitions of OCPRI into the physiologically active state OCPR. F Relative concentration kinetics of the states generated by a 26 fs pulse in OCPWW and corresponding species-associated spectra (SAS) of the states (G) obtained from target analysis of transient absorption spectra of OCPWW considering the model (E).