Abstract
The COVID-19 vaccination programme commenced in England on 8th December 2020 primarily based on age; by 7th March 2021 approximately 93% of the English population aged 70+ years had received at least 1 dose of either the Pfizer BioNTech or AstraZeneca vaccines. Using a nucleoprotein assay that detects antibodies following natural infection only and a spike assay that detects infection and vaccine-induced responses, we aim to describe the impact of vaccination on SARS-CoV-2 antibody prevalence in English blood donors.
Key words: COVID-19, SARS-CoV-2, Antibodies, COVID-19 Serological Testing, COVID-19 vaccines, Surveys, Blood Donors
Letter to the editor
We read with interest in this journal the letter of Tré-Hardy et al.1 which contrasts serological responses following mRNA vaccination in individuals with and without prior infection; good responses were seen in all study participants. England introduced a mass vaccination programme against COVID-19 on 8th December 2020 primarily based on age, starting with those over 80 years of age, along with health and social care workers.2 Since the beginning of the programme to 7th March 2021 over 19 million individuals in England have been vaccinated with at least one dose of vaccine: either Pfizer BioNTech (from 8th December) or AstraZeneca (from 4th January).3 We describe the impact of vaccination rollout on antibody prevalence in blood donors in England.
As part of COVID-19 infection monitoring, Public Health England, in collaboration with the National Health Service Blood and Transplant Service has arranged regular collections of plasma from English blood donors to be sent for COVID serology testing; results are reported weekly.4 Approximately 250 samples per week are collected from each of seven NHS regions. We present seropositivity estimates from 23rd November 2020 onward, which covers the period of vaccine rollout and the peak of England's B.1.1.7-variant dominated epidemic wave.
The vaccination status of donors is not available but parallel testing using a nucleoprotein (Roche N) and a spike (Roche S) assay allows us to monitor trends in natural infection transmission and vaccine-induced seropositivity. Nucleoprotein assays (Roche N) only detect antibodies post natural infection, whereas spike assays (Roche S) detect both post natural infection and vaccine-induced antibodies. Antibody responses to both targets reflect infection/vaccination occurring 2–3 weeks previously given the time taken to generate a SARS-CoV2 antibody response.5 We have shown strong agreement between serological responses using these two assays following natural infection that was sustained 6 months post infection.6
Seropositivity estimates are calculated on a 4-week rolling basis and are population weighted by NHS region, age group and sex. Estimates are not adjusted for assay sensitivity and specificity, which are estimated to be in excess of 97% and 99.8% respectively.7 , 8 Additionally, estimates are compared against vaccine uptake, which is calculated using the National Immunisation Management System (NIMS), a new national vaccine register to facilitate management of the vaccination programme in England.
7720 samples were available during the most recent 4-week period 22nd February-21st March 2021, of which 3224/7720 were Roche S positive and 1111/7717 were Roche N positive. Overall population weighted seropositivity amongst blood donors was 46.4% (95% CI 45.4% - 47.5%) using the Roche S assay. This compares with all-England seropositivity of 54.7%3 (95% CrI 49.3% - 60.5%) from the UK Office of National Statistics (ONS) Infection Survey for the period 18th February – 14th March, based on a single spike target based assay.9 Roche N seropositivity was considerably lower at 14.5% (95% CI 13.7% - 15.4%).
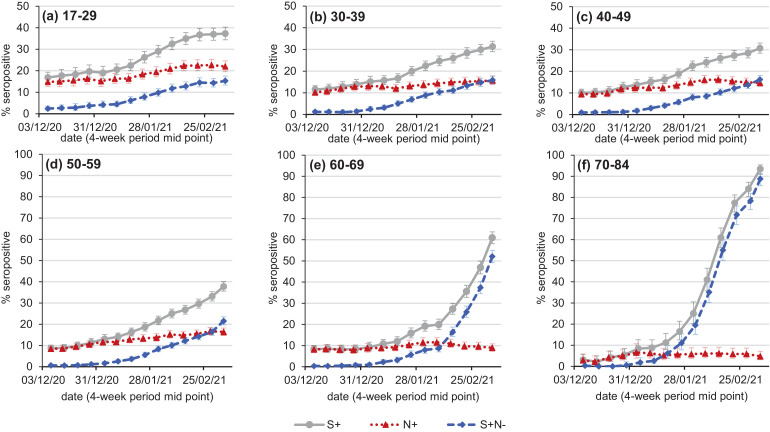
Based on Roche S assay results, seroprevalence has been clearly increasing across all age groups from survey weeks 7th December 2020 – 3rd January 2021 (Fig. 1 ). For the most recent 4-week period, the population weighted seroprevalence was highest in the age 70–84 group at 93.5% (95% CI 90.9% - 95.4%). In parallel, the Roche N assay, a marker for natural infection, showed not only the lowest seroprevalence in the age 70–84 group for the same period at 4.7% (95% CI 3.1% - 7.1%), but this also stabilised over successive four week intervals; for example over the period 1st-31st January 2021 seropositivity was 5.2% (95% CI 3.1% - 8.5%). Seropositivity based on Roche N was highest in the youngest donor cohort and continues to increase, suggesting transmission was ongoing.
Fig. 1.
SARS-CoV-2 antibody seropositivity based on the Roche S assay (S+, grey solid lines), the Roche N assay (N+, red dotted lines) in English blood donors by age group, weighted by NHS region and sex, rolling four weekly average from the 4 week period 25/11/2020 - 20/12/2020 to the 4 week period 22/02/2021 – 21/03/2021. Also shown is the percentage Roche S seropositive, Roche N seronegative (S+N-, blue dashed lines).
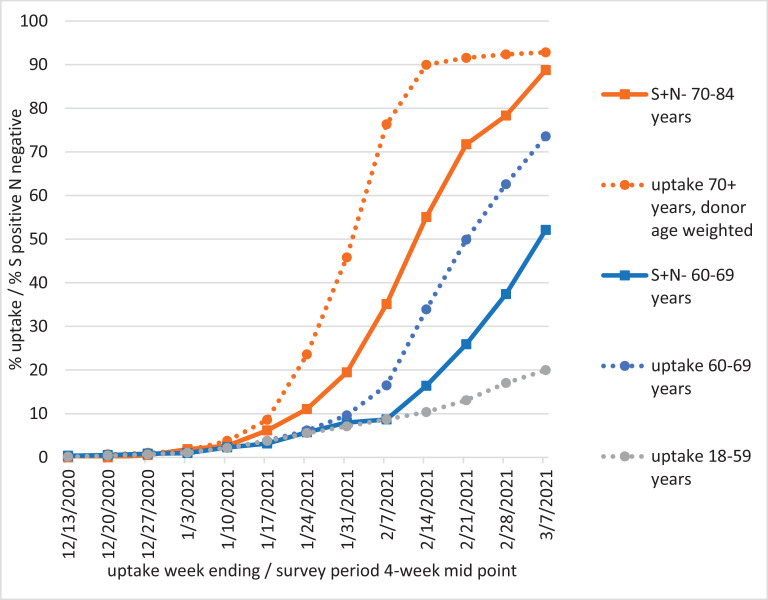
Cumulative first dose vaccine uptake was 91.6% to the week ending 21st February, which roughly corresponds with the most recent 4-week period given 2–3 weeks for antibody response (Fig. 2 ). The increase in S positive N negative outcomes accelerated from survey weeks 11th January – 7th February 2021 following a rise in uptake. Note that age 70+ uptake in Fig. 2 is weighted by the 70+ donor age distribution, which tails off with age.
Fig. 2.
Cumulative dose 1 COVID-19 vaccine uptake by age group (age on 31/03/2021). Roche S positive, N negative is plotted for the 70–84 and 60–69 age groups to demonstrate the lag in antibody response. Age 70+ uptake is weighted by the 70–84 donor age distribution.
The vaccine uptake of 8.7% to the week ending 7th February in those 18–59y is lower than S positive N negative seroprevalence in younger blood donors, suggesting that health and social care workers are over-represented in the latter group.
Since vaccine rollout commenced Roche S seropositivity has increasingly risen above Roche N seropositivity and clearly shows trends in vaccine-induced antibodies, especially within the 70–84 age group who were amongst the first to be targeted for vaccination. Second dose coverage is less an 1% amongst the oldest donor age group, hence we observe a robust antibody response following a single vaccine dose. Meanwhile Roche N seropositivity in this age group has remained stable, suggestive of vaccine impact. This adds to a growing body of evidence suggestive of vaccine impact in the UK population.10
Ethics
PHE has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to process patient information for national surveillance of communicable diseases. Specific ethical approval was not required for this surveillance work.
Author's contributions
HW, SE, IH, SR, KB and GA wrote the manuscript, with input from MR. GA, KB and MR contributed to conceptualization, funding acquisition and project administration. HW performed statistical analysis. EC, AL, CT, CC collated vaccine uptake statistics. EL, IH, SR, JH curated and managed serology data. CR, AO, TB performed the testing. All authors read and approved the submission.
Funding was provided through Public Health England.
Declaration of Competing Interest
EL reports the Public Health England Vaccine Evaluation Unit performs contract research on behalf of GSK, Sanofi and Pfizer which is outside the submitted work.
HW, SE, IH, SR, KB, GA, EC, AL, CT, CC, IH, SR, JH, CR, AO, TB, MR report no conflicts of interest.
Acknowledgement
We are grateful to Su Brailsford and colleagues in NHS Blood and Transplant for the support and collaboration on this work.
References
- 1.Tré-Hardy M., Cupaiolo R., Papleux E., Wilmet A., Horeanga A., Antoine-Moussiaux T., Della Vecchia A., Beukinga I., Vekemans M., Blairon L. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J Infect. 2021 doi: 10.1016/j.jinf.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health and Social Care. Statement from the UK Chief Medical Officers on the prioritisation of first doses of COVID-19 vaccines. 30 December 2020 [cited 2021 Mar 07]. Available from: https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-officers-on-the-prioritisation-of-first-doses-of-covid-19-vaccines.
- 3.The official UK Government website for data and insights on Coronavirus (COVID-19). Vaccinations in United Kingdom. [cited 2021 Mar 16]. Available from: https://coronavirus.data.gov.uk/details/vaccinations.
- 4.Public Health England. National flu and COVID-19 surveillance reports. Week 13 report (up to week 12 data), 1 April 2021 [cited 2021 Apr 01]. Available from: https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports.
- 5.Subbarao S., Warrener L., Hoschler K., Perry K., Shute J., Whitaker H. Robust Antibody Responses in 70-80 Year Olds following 1 or 2 Doses of Pfizer COVID-19 Vaccine. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.12.2100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris R.J., Whitaker H.J., Andrews N.J., Aiano F., Amin-Chowdhury Z., Flood J. Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers. J Infect. 2021;82:162–169. doi: 10.1016/j.jinf.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison, Lancet Infect Dis 2020;20:P1390-1400, 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed]
- 8.Roche Diagnostics. Elecsys® Anti-SARS-CoV-2 S. F. Hoffmann-La Roche Ltd. 2021 [cited 2021 Mar 07]. Available from: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html.
- 9.Office for National Statistics. Coronavirus (COVID-19) Infection Survey antibody data for the UK: 30 March 2021 [cited 2021 Apr 01]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveyantibodydatafortheuk/30march2021.
- 10.Wise J. Covid-19: is vaccination roll out reducing cases and deaths in the UK? BMJ. 2021;372:n506. doi: 10.1136/bmj.n506. https://www.bmj.com/content/372/bmj.n506 Available from: [DOI] [PubMed] [Google Scholar]