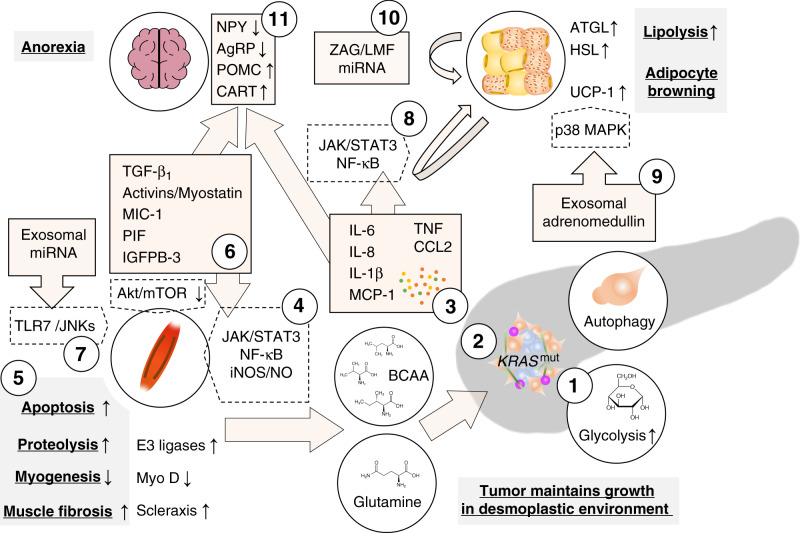
Fig. 2. Tumour-derived factors associated with cachexia in pancreatic adenocarcinoma.
Tumours increasingly metabolise glucose through glycolysis which is increased through mutant KRAS-dependent upregulation of glycolytic enzymes (1). Other KRAS-dependent metabolic changes promote the use of other carbon sources such as glutamine and branched chain amino acids (BCAA) from the breakdown of peripheral tissue, as well as other non-essential amino acids from pancreatic stellate cell autophagy, in the TCA cycle; substrates from the TCA cycle are used for biosynthesis (2). Together, these changes increase survival in a hypoxic and nutrient-deficient environment. Tumour-derived cytokines prompt several catabolic effects in peripheral tissues (3). Interleukin (IL)-6, TNF or interferon γ (IFNg) induce the breakdown of muscle fibres through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT3) pathway and nuclear factor (NF)-κB-dependent induction of nitric oxide (4) which leads to decreased myogenesis through downregulation of the myogenic regulatory factor MyoD and increased proteolysis through E3-ligase-dependent ubiquitination (5). Similarly, members of the transforming growth factor (TGF)-β superfamily activate the canonical Smad2/3 pathway which promotes ubiquitin ligase-mediated proteolysis and scleraxis-mediated muscle fibrosis and reduces Akt/mTOR-mediated myogenesis (6). Additionally, microRNAs (miRNAs) in tumour-derived extracellular vesicles induce myoblast apoptosis by Toll-like receptor 7-dependent c-Jun N-terminal kinase (JNK) signalling (7). In white adipose tissue, pro-inflammatory signals, specifically IL-6, promote lipolysis through activation of the JAK/STAT3 pathway and NF-kB in adipose tissue and induces adipocyte browning through upregulation of uncoupling protein 1 (UCP-1) (8). Lipolysis through hormone-sensitive lipase (HSL) are also mediated by adrenomedullin from tumour-derived exosomes which activates the p38 mitogen-activated protein kinase (MAPK) pathway in adipocytes. Exosomal cargoes (other than adrenomedullin) can also induce adipocyte browning through UCP-1 expression (9). Lipolysis is maintained through paracrine signals in fat tissue liked zinc-α2-glycoprotein (ZAG/LMF) and miRNAs which induce adipose triglyceride lipase (ATGL) and HSL catalyse the hydrolysis of stored triglycerides (10). In the central nervous system, pro-inflammatory cytokines, particularly IL-1β, and macrophage inhibitory cytokine (MIC)-1 (a member of the TGF-β superfamily), induce anorexia by reducing anabolic neuropeptide Y (NPY)/agouti-related protein (AgRP) and increasing catabolic proopiomelanocortin (POMC) and cocaine-and-amphetamine regulated transcript (CART) signalling (11).