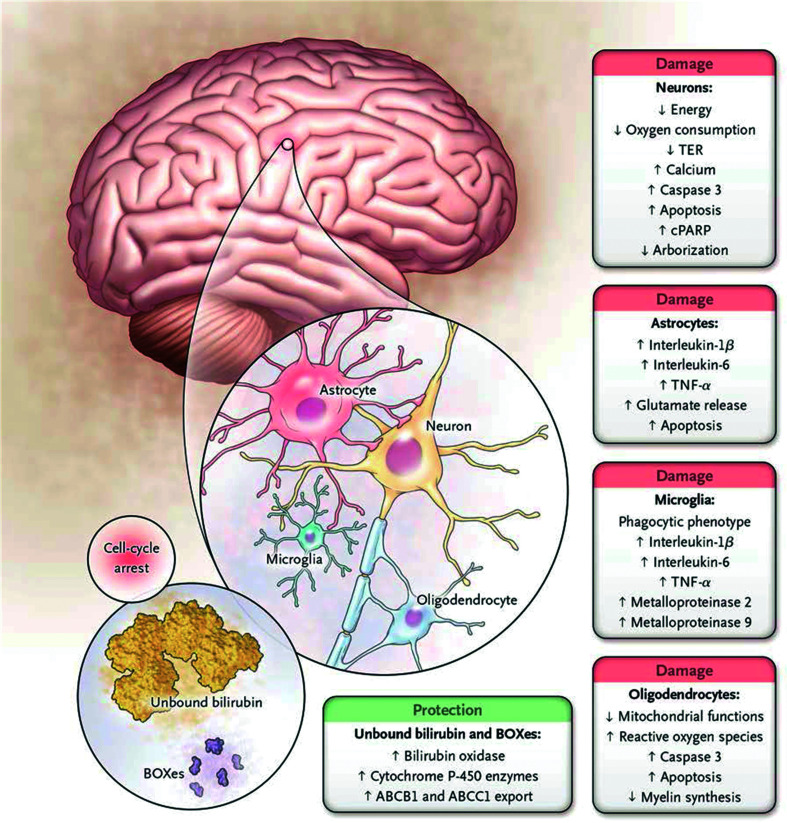
Fig. 5. Cell types and metabolic processes affected by bilirubin in the central nervous system.
The main effects of bilirubin on neurons are decreased oxygen consumption and increased release of calcium and caspase 3, resulting in apoptosis. There is also decreased dendritic and axonal arborization. A similar pattern is observed in oligodendrocytes with increased apoptosis, impairment of the redox state (oxidative stress), and reduced synthesis of myelin. Microglia react to toxic injury associated with bilirubin by increased release of proinflammatory cytokines and metalloproteinase activity as cells manifest a phagocytic phenotype. A similar proinflammatory pattern is observed in astrocytes, with enhanced release of glutamate and apoptosis. At the same time, cells may reduce the intracellular concentration of bilirubin either by extruding the pigment through the ATP-binding cassette transporters or by increasing the formation of the less toxic products through bilirubin oxidation products (BOXes) and/or cytochrome P-450 enzymes (1a1 and 1a2, in particular). These responses are protective, whereas all others result in cell damage; this suggests that once the intracellular concentration of bilirubin exceeds a toxic threshold (still to be defined), the polymorphic metabolic cascade leading to neurotoxicity ensues. (From Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage-mechanism and management approaches. N Engl J Med 2013; 369: 2025). Abbreviations: cPARP, cleaved poly (adenosine diphosphate-ribose) polymerase; TER, transcellular resistance.