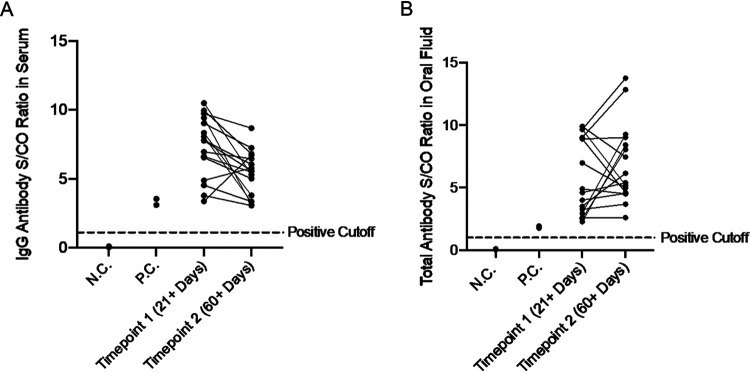
FIG 3.
(A) Detection of antibodies against SARS-CoV-2 using the EuroImmun COVID-19 antibody detection kit in participant-derived serum samples collected from participants who previously tested positive for COVID-19 by an oral-fluid PCR test both 21+ days post-symptom onset and 60+ days post-symptom onset. All samples passed the positive cutoff value of 1.1 set by the manufacturer (represented by the horizontal dashed line). N.C. = negative control; P.C. = positive control. ROC analysis of this data set in serum resulted in 100% sensitivity and 100% specificity with an area under the ROC curve (AUC) of 1. (B) Detection of antibodies against SARS-CoV-2 in oral fluid samples collected using the OraSure Technologies oral fluid specimen collection device from the same participants whose serum was tested. The manufacturer-provided positive cutoff value of 1.0 is represented by the dotted line. N.C. = negative control; P.C. = positive control. ROC analysis of this data set in oral fluid revealed 100% sensitivity and 100% specificity with the manufacturer-provided cutoff value of 1.0 for total antibody units and an AUC of 1.