Figure 1. The KMO reaction cycle.
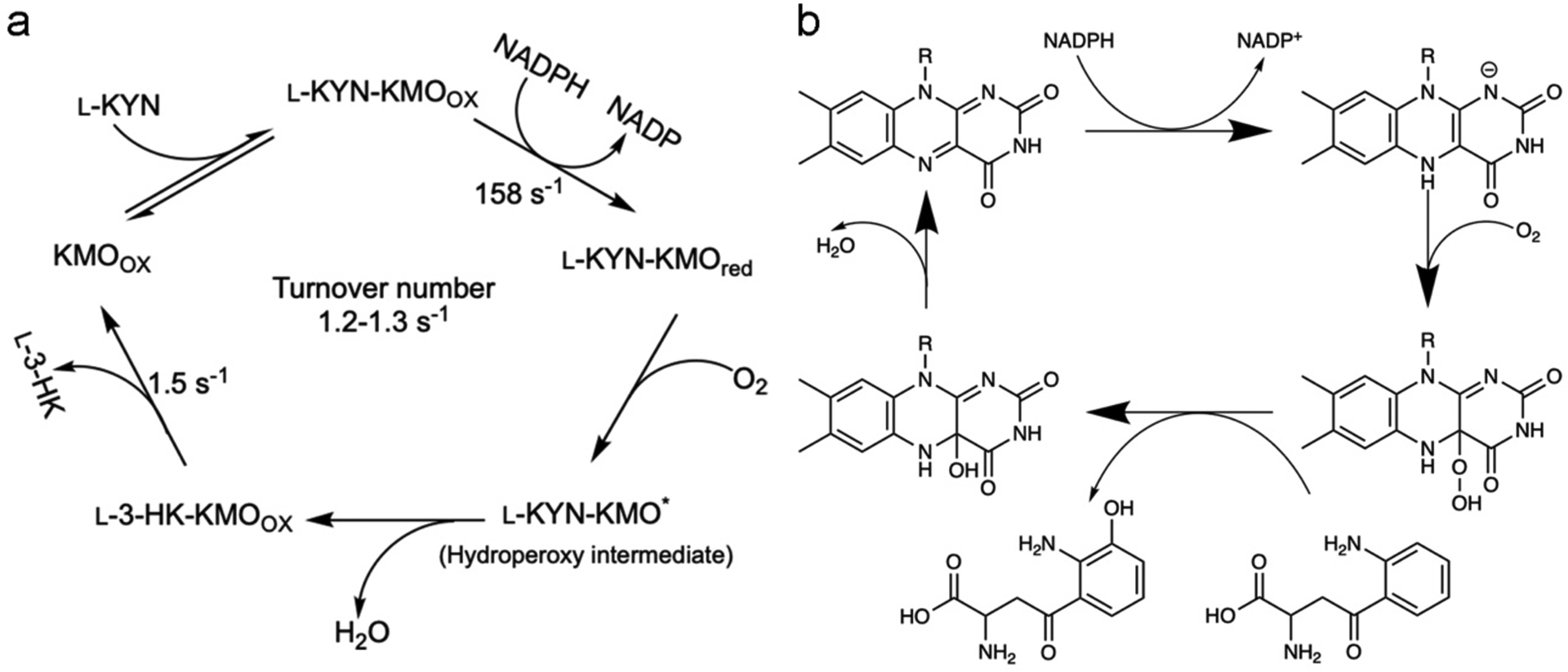
In the KMO reaction, L-KYN binds to the protein first, then NADPH binds and reduces the FAD followed by NADP+ release, allowing for dioxygen binding. A FAD–superoxide radical pair is likely to be formed and rapidly decays to a peroxy-flavin structure, which will act as the electrophile for L-KYN oxidation. C4a-hydroxy flavin is then dehydrated yielding the oxidised flavin. The final step is 3-HK release, which is also the rate limiting step with a slightly faster rate than the overall turnover number [56].
