Abstract
Objectives:
In this study, we aimed to (1) estimate the severe acute respiratory coronavirus 2 (SARS-CoV-2) infection rate and the secondary attack rate among healthcare workers (HCWs) in Québec, the most affected province of Canada during the first wave; (2) describe the evolution of work-related exposures and infection prevention and control (IPC) practices in infected HCWs; and (3) compare the exposures and practices between acute-care hospitals (ACHs) and long-term care facilities (LTCFs).
Design:
Survey of cases.
Participants:
The study included Québec HCWs from private and public institutions with laboratory-confirmed coronavirus disease 2019 (COVID-19) diagnosed between March 1 and June 14, 2020. HCWs aged ≥18 years who worked during the exposure period and survived their illness were eligible for the survey.
Methods:
After obtaining consent, 4,542 HCWs completed a standardized questionnaire. COVID-19 rates and proportions of exposures and practices were estimated and compared between ACHs and LTCFs.
Results:
HCWs represented 13,726 (25%) of 54,005 reported COVID-19 cases in Québec and had an 11-times greater rate of COVID-19 than non-HCWs. Their secondary household attack rate was 30%. Most affected occupations were healthcare support workers, nurses and nurse assistants working in LTCFs (45%) and ACHs (30%). Compared to ACHs, HCWs in LTCFs had less training, higher staff mobility between working sites, similar PPE use, and better self-reported compliance with at-work physical distancing. Suboptimal IPC practices declined over time but were still present at the end of the first wave.
Conclusion:
Québec HCWs and their families were severely affected during the first wave of COVID-19. Insufficient pandemic preparedness and suboptimal IPC practices likely contributed to high transmission in both LTCFs and ACHs.
Healthcare workers (HCWs) have been at high risk of coronavirus disease 2019 (COVID-19) all over the world. As of July 2020, the proportion of HCWs infected among all COVID-19 cases varied between countries: 4.2% in China, 12% in Italy, 16% in Spain, 17.5% in Ontario, Canada, 17.8% in the United States, and 18% in France.1-5 In Canada, 53% of all cases during the spring of 2020 were reported by the Province of Québec, which has 23% (8.4 million) of the Canadian population.6 In Québec, 25% of all confirmed COVID-19 cases were HCWs. Early in the pandemic, lack of clinical and epidemiological data, shortage of protective equipment, insufficient testing capacity (leading to prioritization of HCWs), delays in communication, and unpreparedness for epidemic response have been cited among the main reasons for the excess burden of COVID-19 in HCWs.7,8 But all of these factors have been in constant evolution, as reflected by the ever-evolving infection prevention and control (IPC) guidelines during the first wave.7
In the few studies that have examined the HCW characteristics, the exposures and the IPC practices associated with the risk of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infections in healthcare settings occurred in acute-care hospitals (ACHs). These studies had mostly cross-sectional designs and did not show the changes in protective measures and incidence of infection in the context of evolving local epidemics.9-12 Limited information is available regarding HCWs in long-term care facilities (LTCF) who were severely affected by the COVID-19 epidemic worldwide.13,14
In this large study, we aimed to (1) estimate infection rates and secondary household transmission of Québec HCWs during the spring 2020 wave of COVID-19, (2) describe evolution of work-related exposures and IPC practices of SARS-CoV-2 infected HCWs, and (3) compare these exposures and practices between ACHs and LTCFs.
Methods
Study population
This survey of cases, conducted after the standard case investigation by public health, included any HCW, defined as a care provider and/or working in a healthcare setting in Québec province, who had a COVID-19 polymerase chain reaction (PCR)–confirmed diagnosis between March 1 and June 14, 2020. These HCWs were identified from the provincial database of all COVID-19 cases reported to public health, which included the HCW status identified during the public health investigation. Although data on all HCWs were used to estimate the global burden, only those aged ≥18 years, speaking French or English, having worked during the exposure period (defined as 3–10 days before symptoms onset or testing date) and, having survived their illness, were eligible for the survey.
Data collection
HCWs were contacted between May 6 and June 22, and eligible HCWs who agreed to participate were asked to complete a 30-minute phone or online questionnaire. The questionnaire was developed by the research team which included infectious disease, IPC and occupational health specialists (Supplementary Material online).
Participants were queried about their sociodemographic characteristics, their perceived likely source of exposure (workplace, household, travel, community, etc) and their job characteristics (occupation, facility, department, patient facing contacts). Questions about exposure to COVID-19 patients or colleagues and IPC practices during the exposure period were also asked, including details about personal protective equipment (PPE) worn when contacting nonsuspected COVID-19 patients, COVID-19 patients, and compliance with PPE doffing protocol. Additional data were collected regarding household size, the number of household members who developed COVID-19–compatible symptoms, and the first symptomatic person in the household according to the participant.
Data analysis
The numbers of all reported new infections from March 1 to June 14 were used to estimate cumulative incidences and 95% confidence intervals for HCWs and for non-HCWs aged 20–69 years. Numerators for health occupation rates were estimated assuming the same proportions in all HCWs as among survey participants. Denominators were taken from the 2016 Canadian Census, increased by 5% to account for the annual growth, and from the College of Physicians of Québec and the Québec Orders of Nurses and Nurse Assistants.15-18
Secondary household attack rates were estimated in a subsample of 3,823 participants who lived in households with ≥2 members where the HCW was the first case.
Proportions or median and full range were calculated for each variable, and prevalence of IPC practices were estimated by type of patient exposition. The evolution of COVID-19 prevention and control measures was plotted by CDC week of symptom onset or testing if asymptomatic and trends were tested using a Cochrane-Armitage test. Changes were described for early spring (March 1–April 11), mid spring (April 12–May 16) and late spring (May 17–June 14). Characteristics, exposures and practices in ACHs and LTCFs, including participants working exclusively in one type of facility, were statistically compared using χ2 tests.
Ethical aspects
This study was conducted under the legal mandate awarded by the National Director of Public Health of Québec under the Public Health Act. It was approved by the research ethics committee of the CHU de Québec–Université Laval. Verbal consent during phone interviews and/or electronic consent was obtained from all participants.
Results
Burden of COVID-19 among HCWs
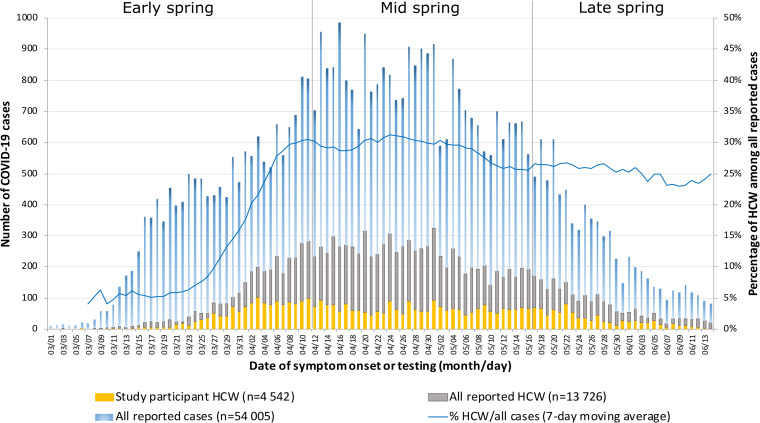
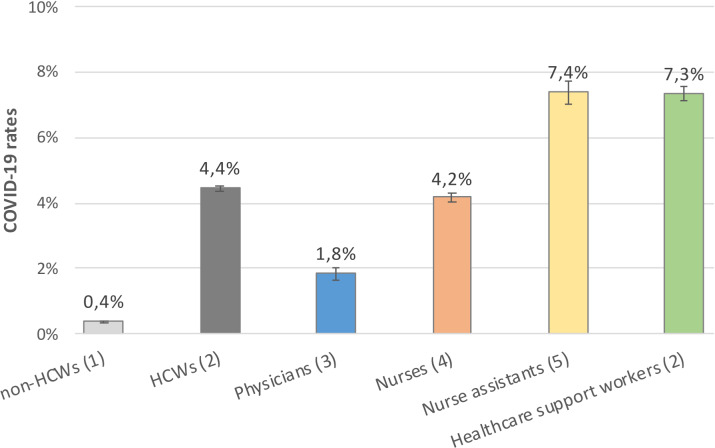
In the provincial database, 54,005 confirmed COVID-19 cases occurred between March 1 and June 14, 2020, and 13,726 (25.4%) of these were HCWs. This proportion increased from 19.6% in early spring to 28.7% in mid-spring and declined to 25.5% by late spring (Fig. 1). The estimated rate of reported COVID-19 was 11 times greater in HCWs compared to non-HCWs aged 20–69 years (4.4% vs 0.4%). By occupation, the COVID-19 rate was highest among healthcare support workers (7.3%) and nurse assistants (7.4%), and it was lower in nurses (4.2%) and physicians (1.8%) (Fig. 2).
Fig.1.
Number and percentage of reported COVID-19 cases, reported infected healthcare workers and participants to the study per day of symptom onset (or day of testing) and proportion of healthcare workers among all reported infections.
Note. HCW, healthcare worker.
Fig. 2.
COVID-19 rates and 95% confidence intervals for general population non-healthcare workers aged 20 to 69 years old (excluding healthcare workers), for healthcare workers and for some health occupations in Québec, March 1–June 14, 2020. Note. HCW, healthcare worker; public, working for the public health system. Source of denominators to estimate different rates:1Québec Statistic Institute, population of 2019.2Statistics Canada, 2016 Census (with an added 5% growth of HCW according to other sources).3College of Physicians of Québec, data updated in 2019.4Québec Order of Nurses, data updated 2019.5Québec Order of Nurse Assistants, data updated 2019.
Survey participants
Among the 11,223 HCWs who were called, 8,482 (75.6%) were reached: 986 (11.6%) refused to participate, 198 (2.3%) did not meet the inclusion criteria (44 not a HCW, 58 not a COVID-19 case, 14 not English or French speakers, and 82 other reasons), 2,224 (26.2%) agreed to participate but did not complete the online questionnaire and 532 (6.3%) did not work during the exposure period. Thus, 4,542 participants remained for the analysis (Supplementary Fig. 1 online). Based on the information from the provincial database, sociodemographic characteristics, probable source of infection, occupation, and workplace were similar between participant and nonparticipant HCWs (data not shown).
Sociodemographic and work characteristics
Among the 4,542 participants, 92.3% reported the presence of symptoms, 79.2% were female, and the median age was 42 years. For the type of HCW, 35.1% were healthcare support workers (providing basic personal care like cleaning, dressing and feeding patients), 22.6% were nurses, 11.9% were nurse assistants, 6.0% were managers or administrative workers, and 3.1% were physicians (Table 1).
Table 1.
Demographic and Employment Characteristics and Workplace Exposures to COVID-19 by Type of Facility
| Characteristic | Type of Facility | ||||
|---|---|---|---|---|---|
| Total No. (%) |
ACH No. (%)a |
LTCFa
No. (%) |
Otherb
No. (%) |
Severalc
No. (%) |
|
| 4542 | 1,386 (30.5) | 2,058 (45.3) | 903 (19.9) | 195 (4.3) | |
| Age, median Y (full range) | 42 (18–82) | 39 (18–82) | 44 (18–74)d | 44 (19–73) | 38 (18–67) |
| Sex, female | 3,624 (79.8) | 1,099 (79.3) | 1,646 (80.0) | 726 (80.4) | 153 (78.5) |
| Presence of COVID-19 symptoms | 4,191 (92.3) | 1,301 (93.9) | 1,862 (90.5)cd | 846 (93.7) | 182 (93.3) |
| Profession | d | ||||
| Healthcare support workers | 1,594 (35.1) | 267 (19.3) | 992 (48.2) | 300 (33.2) | 35 (18.0) |
| Nurses | 1,024 (22.6) | 514 (37.1) | 325 (15.8) | 137 (15.2) | 48 (24.6) |
| Nurse assistants | 541 (11.9) | 151 (10.9) | 286 (13.9) | 91 (10.1) | 13 (6.7) |
| Administration/Managers | 273 (6.0) | 89 (6.4) | 89 (4.3) | 85 (9.4) | 10 (5.1) |
| Housekeeping | 164 (3.6) | 42 (3.0) | 95 (4.6) | 22 (2.4) | 5 (2.6) |
| Physicians | 140 (3.1) | 67 (4.8) | 14 (0.7) | 18 (2.0) | 41 (21.0) |
| Other types of work | 806 (17.7) | 256 (18.5) | 257 (12.5) | 250 (27.7) | 43 (22.1) |
| Public facility e | 3,684 (81.1) | 1,366 (98.6) | 1,703 (82.8)d | 425 (47.1) | 190 (97.4) |
| Mobility | |||||
| Work in >1 facility habitually | 1,161 (25.6) | 222 (16.0) | 530 (25.8)d | 214 (23.7) | 195 (100) |
| Work in >1 facility during exposure period | 477 (10.5) | 56 (4.0) | 170 (8.3)d | 86 (9.5) | 165 (84.6) |
| Work in ≥3 facilities during exposure period | 107 (2.3) | 8 (0.6) | 46 (2.2)d | 18 (2.0) | 35 (17.9) |
| Work in >1 department during exposure period | 516 (11.4) | 285 (20.6) | 84 (4.1)d | 70 (7.8) | 77 (39.5) |
| Work shift | d | ||||
| Day (8:00 h–16:00 h) or day/evening (8:00 h–24:00 h) | 2,853 (62.8) | 809 (58.4) | 1,288 (62.6) | 629 (69.7) | 127 (65.1) |
| Evening (16:00 h–24:00 h) | 781 (17.2) | 254 (18.3) | 399 (19.4) | 110 (12.2) | 18 (9.2) |
| Night (00:00 h–8:00 h) or evening/night (16:00 h–8:00 h) | 491 (10.8) | 117 (12.8) | 226 (11.0) | 75 (8.3) | 13 (6.7) |
| Rotating shift | 417 (9.2) | 146 (10.5) | 145 (7.1) | 89 (9.9) | 37 (19.0) |
| Exposure | |||||
| Contact with COVID-19 infected coworkers | 2,582 (56.8) | 767 (55.3) | 1,224 (59.5)d | 489 (54.2) | 102 (52.3) |
| Contact with patients | 4,047 (89.1) | 1,214 (87.6) | 1,938 (94.2)d | 720 (79.7) | 175 (89.7) |
| Contact with COVID-19 patients | 3,411 (75.0) | 1,037 (74.8) | 1,699 (82.6)d | 527 (58.4) | 148 (75.9) |
| Nonexclusive COVID-19 unit | 2,160 (63.3) | 566 (54.6) | 1,111 (65.4)d | 397 (75.3) | 86 (58.1) |
| Exclusive COVID-19 unit | 1,251 (36.7) | 471 (45.4) | 588 (34.6)d | 130 (24.7) | 62 (41.9) |
| Exclusive COVID-19 ICU unit | 83 (2.4) | 73 (7.0) | 0 (0.0) | 5 (1.0) | 5 (3.4) |
| Emergency department | 226 (6.6) | 193 (18.6) | 0 (0.0) | 8 (1.5) | 22 (14.9) |
| Direct care to COVID-19 patients | 2,822 (82.7) | 844 (81.4) | 1,446 (85.1)d | 414 (78.6) | 118 (79.7) |
| Attendance at AGMPs | 136 (4.0) | 93 (9.0) | 25 (1.5)d | 7 (1.3) | 7 (4.7) |
Note. ACH, acute-care hospital; LTCF, long-term care facility; ICU, intensive care unit; AGMP, aerosol-generating medical procedure.
Healthcare workers with exclusive work in acute-care hospitals or long-term care facilities.
Other reported facilities: private residences for elderly, community health centers, other residential facilities (care homes and assisted-living facilities), rehabilitation centers, private medical clinics, COVID-19 clinics, ambulances, laboratories, pharmacies, public health department, others.
Several types of facilities. For example, if working in several LTCFs, they were classified as LTCF.
Test χ2 comparing acute-care hospitals versus long-term care facilities statistically significant (P < .05).
All facilities where participant works are public.
Among participants, 48.2% worked in LTCFs (45.3% exclusively in LTCFs), 34.2% worked in ACHs (30.5% exclusively in ACHs), 9.5% worked in private residences for elderly, 5.6% worked in public community health centers, 4.8% worked in other residential facilities (care homes and assisted-living facilities), 4.4% worked in rehabilitation centers, 3.1% worked in private medical clinics, and 10.8% worked in other settings. Although 25.6% reported usually working in >1 facility, 10.5% did so during their exposure period, and this rate was twice as high in LTCF workers (8.3%) as it was in ACH workers (4.0%) (Table 1).
In LTCFs, most participants were healthcare support workers (48.2%), followed by nurses (15.8%) and nurse assistants (10.9%). In ACHs, most participants were nurses (37.1%), followed by healthcare support workers (19.3%), and nurse assistants (11.9%) (Table 1). Among the 1,386 HCWs exclusively working in ACHs, 37.5% worked in medical units, 18.3% worked in surgical units, 16.0% worked in emergency departments, 14.4% worked in geriatric departments, and 8.4% worked in intensive care units. Also, 20.6% worked in >1 department.
Secondary household transmission
In the 3,823 households analyzed, COVID-19 symptoms developed in 2,718 (29.8%) of 9,096 other household members, 1,915 (34.5%) of 5,543 adults, and 803 (22.6%) of 3,553 children <18 years old. The secondary attack rate decreased with larger number of household members from 39.9% in households of 2 members to 22.5% if ≥7 members. Overall, 55.8% of households had no secondary cases, 23.0% had all other members becoming sick, and 21.2% had some symptomatic members but not all (Table 2).
Table 2.
Number and Proportions of Households According to the Number of Secondary COVID-19 Cases and Secondary Attack Rate by Household Size
| No. of Persons Living With the COVID-19 HCW | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | ≥6 | Total (≥1) | ||
| Households, no. (%) | 1,335 | 809 | 984 | 442 | 156 | 97 | 3,823 | % of All Infected Persons | |
| Secondary cases in the household, no. (%) | 0 | 803 (60.1) | 476 (58.8) | 518 (52.6) | 217 (49.1) | 76 (48.7) | 44 (45.4) | 2,134 (55.8) | 0 |
| 1 | 532 (39.9) | 179 (22.1) | 201 (20.4) | 85 (19.2) | 30 (19.2) | 18 (18.6) | 1,045 (27.3) | 38.4 | |
| 2 | NA | 154 (19.0) | 132 (13.4) | 52 (11.8) | 20 (12.8) | 9 (9.3) | 367 (9.6) | 27.0 | |
| 3 | NA | NA | 133 (13.5) | 43 (9.7) | 13 (8.3) | 7 (7.2) | 196 (5.1) | 21.6 | |
| 4 | NA | NA | NA | 45 (10.2) | 7 (4.5) | 9 (9.3) | 61 (1.6) | 9.0 | |
| 5 | NA | NA | NA | NA | 10 (6.4) | 4 (4.1) | 14 (0.4) | 2.6 | |
| 6 | NA | NA | NA | NA | NA | 5 (5.2) | 5 (0.1) | 1.1 | |
| 7 | NA | NA | NA | NA | NA | 1(1.0)a | 1 (0.0) | 0.3 | |
| Persons, no. | 1,335 | 1,618 | 2,952 | 1,768 | 780 | 643 | 9,096 | ||
| Persons with COVID-19 symptoms, no. | 532 | 487 | 864 | 498 | 187 | 146 | 2,718 | ||
| Secondary attack rate (95% CI) |
39.9% (37%–43%) |
30.1% (28%–32%) |
29.3% (28%–31%) |
28.2% (26%–30%) |
24.0% (21%–27%) |
22.5% (19%–26%) |
29.9% (29%–31%) |
||
Note. NA, not applicable; CI, confidence interval.
The household with 7 secondary cases had 7 persons living with the infected HCW.
Exposure
The workplace was the perceived probable source of infection of 85.4% of participants: 33.7% indicated that their infection was likely transmitted from patients, 9.8% indicated that their infection was likely transmitted from infected coworkers, and 41.9% indicated that they could not determine whether their actual source of infection was patients or coworkers. Other reported sources of infection were household members (3.9%), extended family or other social contacts (0.7%), the community (1.2%), multiple possible sources including the workplace (5.8%), and unknown (2.8%).
Exposure to infected coworkers was reported by 56.9% of participants (Table 1). Exposure to patients with suspected or confirmed COVID-19 was reported by 3,411 (75.1%), of whom 36.7% worked in exclusively dedicated COVID-19 units. Compared with ACHs, HCWs in LTCFs more often had contact with patients and with COVID-19 patients in nonexclusive units (Table 1).
Pandemic preparedness and IPC practices
Overall, 10.9% participants reported having received no IPC training about COVID-19 since February 2020, and 33.6% reported having received only written or posted recommendations. In early spring, no training was reported by 19.0%, and this rate declined to 3.5% in late spring, whereas the percentage who only received written recommendations remained similar (Fig. 3). Absence of training was more frequently reported by healthcare support workers (16.3% vs 8.3% of nurses and 7.5% of all other HCWs; P < .01) and by HCWs working in nonexclusive COVID-19 units (12.6% vs 6.1% for exclusively dedicated COVID-19 units; P < .01). In LTCFs, 13.9% of HCWs had no training, compared with 5.7% in ACHs (P < .01), a difference that persisted in late spring (5.3% vs 1.9%) (Supplementary Fig. 2 and Supplementary Table 1 online).
Fig. 3.
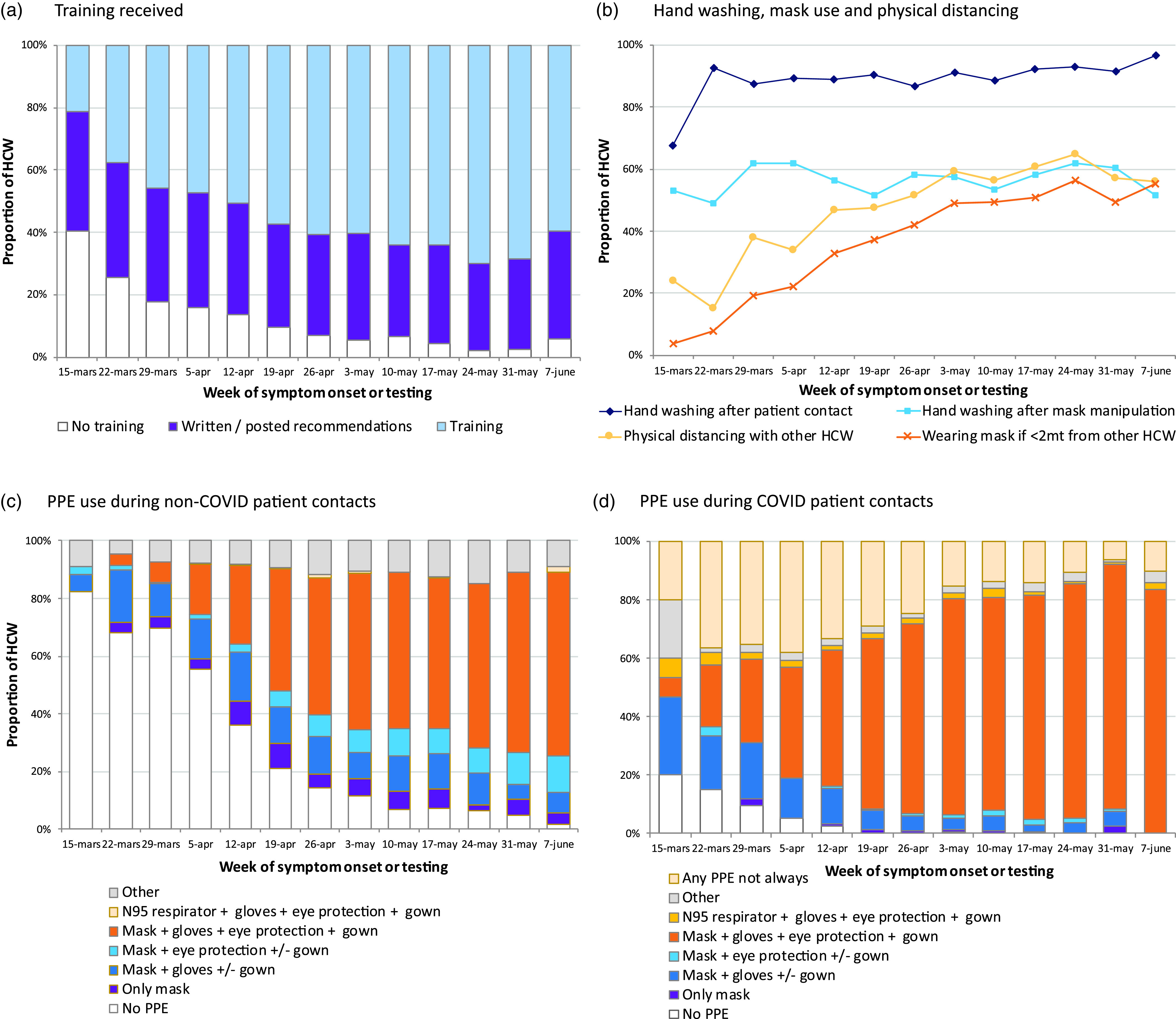
Temporal evolution of the infection prevention and control practices among HCWs infected with COVID-19.
Note. HCW, healthcare worker; PPE, personal protective equipment.
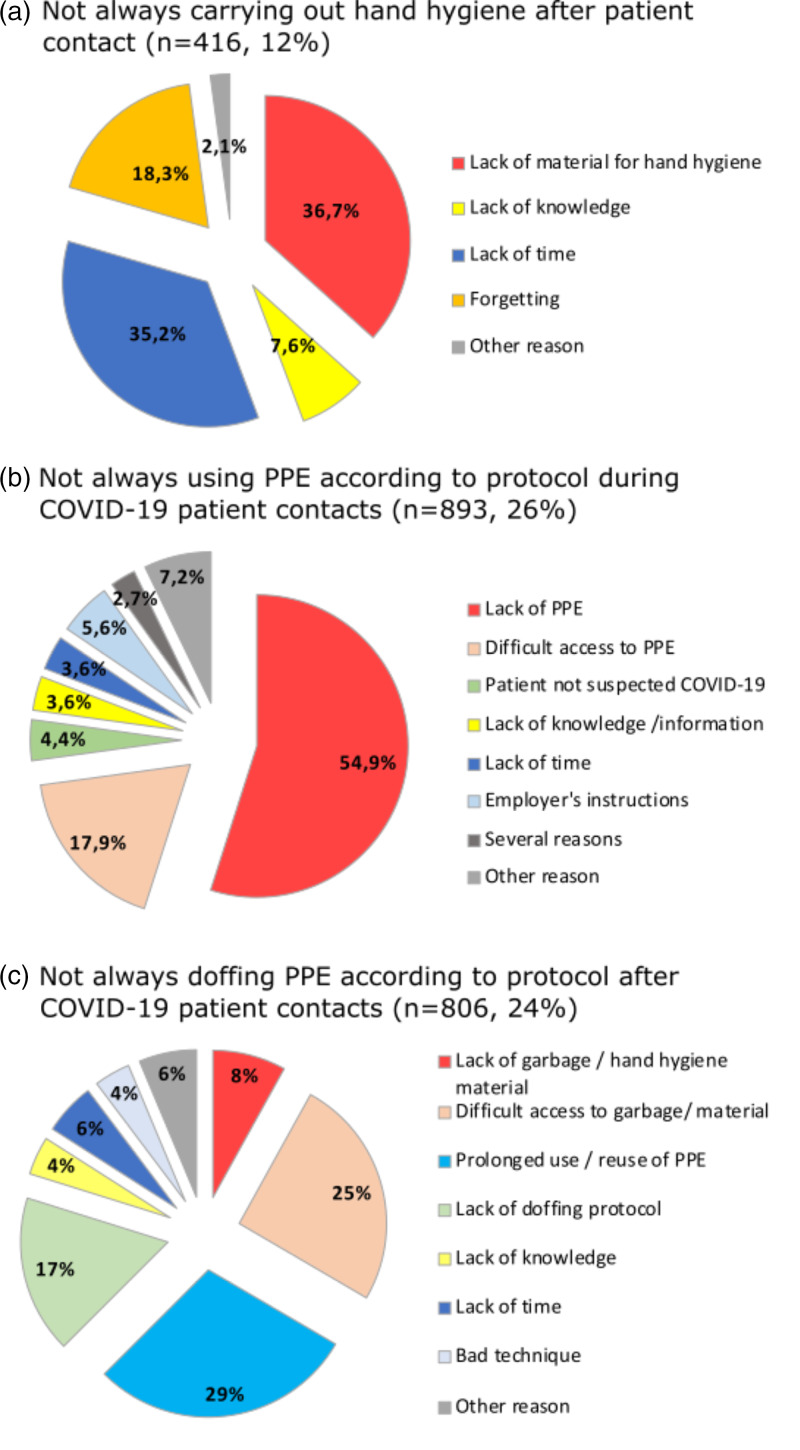
On a self-reported basis, consistent practice of hand hygiene after patient contact was done by 89.4% of participants and slightly improved throughout the study period (P < .01) (Fig. 3). The main reasons for not carrying out hand hygiene were lack of available sink, soap, or hydroalcoholic solution (36.7%); lack of time (35.2%); and forgetting (18.3%) (Fig. 4). Consistent physical distancing during meals increased from 32.6% to 60.8% from early to late spring (P < .01), whereas always wearing a mask when <2 m from coworkers increased from 19.2% to 52.3% during this period (P < .01) (Fig. 3).
Fig. 4.
Reasons for incorrect hand hygiene after patient contact, incorrect PPE use during contacts with COVID-19 patients, and noncompliance with PPE doffing protocol.
Note. PPE, personal protective equipment.
Compared with ACHs, HCWs from LTCFs similarly practiced hand hygiene (88.8% vs 89.3%; P = .75), but they more often kept physical distance (56.3% vs 36.8%; P < .01) or wore masks (56.3% vs 36.8%) during contact with other HCWs (Supplementary Fig. 2 online).
During contacts with non–suspected COVID-19 patients, 29.7% of the 3,699 responders reported not having used any PPE, whereas 66.6% wore a surgical mask and 36.7% used full PPE (ie, mask, ocular protection, gloves, and gown). Full PPE use was more frequent in LTCFs (40.7%) than in ACHs (34.3%; P < .01) (Table 3). Throughout the study period, we detected a significant decrease of HCWs wearing no PPE (P < .01), from 63.7% during early spring to 6.1% in late spring (Fig. 3).
Table 3.
Use of Personal Protective Equipment (PPE) by Type of Exposure to Patients and Type of Facility
| Variable | Type of Patient Exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non–suspected COVID-19 Patient | COVID-19 Patient Excluding AGMPa |
AGMP With COVID-19 Patient |
|||||||
| Frequency | Any frequencyb | Always | Always | ||||||
| Healthcare facility | All | ACH | LTCF | All | ACH | LTCF | All | ACH | LTCF |
| PPE used | |||||||||
| No. of participants | 3,699 | 1,076 | 1,795 | 3,249 | 975 | 1,635 | 131c | 95 | 22 |
| No PPE | 1,100 (29.7) | 313 (29.1) | 553 (30.8) | 74 (2.3) | 13 (1.3) | 45 (2.8)d | 1 (0.8) | 1 (1.1) | 0 (0.0) |
| Surgical mask | 2,462 (66.6) | 725 (67.4) | 1,177 (65.6) | 2,290 (70.5) | 691 (70.9) | 1140 (69.7) | 50 (38.2) | 30 (31.6) | 10 (45.5)d |
| N95 respirator | 111 (3.0) | 66 (6.1) | 21 (1.2)d | 223 (6.9) | 172 (17.6) | 23 (1.4)d | 65 (49.6) | 57 (60.0) | 3 (13.6)d |
| Mask/respirator + eye protection | 1,800 (48.7) | 525 (48.8) | 912 (50.8) | 2,071 (63.7) | 832 (85.3) | 1,199 (73.3)d | 82 (62.5) | 63 (66.3) | 9 (40.9)d |
| Surgical mask + eye protection + gloves + gown | 1,357 (36.7) | 369 (34.3) | 731 (40.7)d | 1,928 (59.3) | 609 (62.5) | 950 (58.1)d | 32 (24.4) | 18 (19.0) | 6 (27.3) |
| N95 respirator + eye protection + gloves + gown | 15 (0.4) | 5 (0.5) | 5 (0.3) | 66 (2.0) | 53 (5.4) | 6 (0.4)d | 46 (35.1) | 43 (45.3) | 2 (9.1)d |
| PPE doffing | |||||||||
| No. of participants | 3,427 | 1,018 | 1,710 | ||||||
| Always doff PPE per protocol | 2,619 (76.4) | 779 (76.5) | 1,312 (76.7) | ||||||
Note. ACH, acute-care hospital; AGMP, aerosol-generating medical procedures; LTCF, long-term care facility; PPE, personal protective equipment.
AGMP: aerosol-generating medical procedures that include aspiration of tracheal secretions in an intubated or tracheotomized patient, autopsy, bronchoscopy, cardiopulmonary resuscitation, child nasopharyngeal suction, manual ventilation before intubation, nasopharyngeal or oropharyngeal surgery, sputum induction, tracheal intubation or extubation.
Frequency of PPE use not reported for contacts with non–COVID-19 patients.
5 participants with no information on the frequency of PPE use.
χ2 test or Fisher exact test comparing acute-care hospitals with long-term care facilities. Statistical significance at P< .05.
During non–aerosol-generating contacts with COVID-19 patients, almost all (97.7%) of the 3,249 participants reported always using some PPE: 70.5% wore a surgical mask and 6.9% wore a N95 respirator. Full PPE with a surgical mask was consistently used by 59.3% of HCWs, slightly lower in LTCFs than in ACHs (58.1% vs 62.5%; P = .03), but much higher among those working in exclusively dedicated COVID-19 units than in nonexclusive units (73.4% vs 50.8%; P < .01). The proportion of participants in full PPE increased over the study period (P < .01), from 32.5% in early to 79.5% in late spring (Fig. 3). The main reported reasons for not always using the recommended PPE were lack of equipment (54.9%), lack of access to equipment (17.9%), and employers’ instructions (5.6%). Lack of and difficult access to PPE were the main reasons for not wearing PPE throughout the study period, both in ACHs (71.6%) and in LTCFs (76.9%) (Fig. 4).
Aerosol-generating medical procedures were attended by 136 (3.0%) participants, of whom only 35.1% always wore the recommended PPE (ie, N95 respirator, eye protection, gloves and gown) (Table 3). Noncompliance with the doffing protocol after contact with COVID-19 patients was reported by 23.6% of participants (Table 3), a proportion that decreased from 27.7% in early spring to 16.7% in late spring. The following reasons were most frequently reported: absence or difficult access to garbage can or hand hygiene material (33.4%), prolonged use or reuse of PPE items (29.1%), and lack of protocol (17.3% and 31.3% in early spring) (Fig. 4).
Discussion
The results of this study show the high risk of COVID-19 among Québec HCWs during the first pandemic wave. Healthcare support workers, nurses, and nurse assistants were the most affected HCWs. Several problems related to pandemic preparedness and IPC measures were identified, many of which were still reported at the end of the first wave: insufficient IPC training, lack of protocols and PPE, high interinstitutional and interdepartmental HCW mobility, and breaches in IPC practices at the individual level. Although training and staff mobility were worse in LTCFs than in ACHs, PPE practice was similar, and compliance with physical distancing and mask use during contact with colleagues was better in LTCFs.
Our results indicate an 11-times higher risk of COVID-19 in HCWs than non-HCW adults, and this is one of the highest risk levels published to date. It is probably overestimated by the greater access to SARS-CoV-2 PCR detection tests of HCWs compared to the rest of the adult population; hospitalized symptomatic patients, LCTF residents, and symptomatic HCWs in contact were prioritized for testing from March to end of May. The testing policy might also have led to underestimation of SARS-CoV-2–infected HCWs, as shown by the low proportion of asymptomatic cases reported (8%), compared to 16% among HCWs from Ontario.5 A prospective online study found an infection rate of 2.7% (2.9% in the United Kingdom and 1.8% in the United States) among frontline HCW participants, 11.6 times higher than in the general community.19 Ontario reported a rate 5.5 times higher for HCWs compared with non-HCWs.5 In Scotland, hospital HCWs had a 3.4 times higher prevalence of SARS-CoV-2 antibodies than age- and sex-matched controls of general population (14.5% vs 4.8%).20 In Wuhan, the attack rates among HCWs were 5.4 times higher than among non-HCWs.21
Overall, 30% of other household members developed COVID-19–compatible symptoms, highly suggestive of COVID-19 given their epidemiological link with a laboratory-confirmed case. This rate might have been underestimated because asymptomatic secondary cases were not detected. This rate is higher than the 17% pooled estimation from a recent meta-analysis of published studies in which secondary attack rates in households varied between 4% and 36%.5 Patient-facing staff, mostly nurses and physicians, have been identified as occupations at highest risk, but most of these studies were hospital based.12,13,22-24 In our study, 35% of all infected HCWs were healthcare support workers (51% in LTCFs), 23% were nurses, 12% were nurse assistants, and 45% worked in LTCFs. These findings are fairly similar to the US data, in which the most affected occupations were healthcare support workers (32%) and nurses (30%) and most (67%) of infected HCWs worked in nursing homes and residential care facilities.14
Among study participants in contact with COVID-19 patients, 37% worked in COVID-19–dedicated units (2% intensive care units) where exposure to COVID-19 is constant but HCWs appeared better trained and seemed to use PPE more rigorously, as shown in the current study. Although some studies have shown that working with COVID-19 patients increased the risk of infection,9,12,25,26 others have described similar risk for those with or without contacts with COVID-19 patients and for those working in high-risk versus low-risk departments.10,27-29
Several factors related to insufficient pandemic preparedness were identified. Nearly half of infected HCWs had either no IPC training (11%) or received only written recommendations (34%). Lack of IPC protocol was frequently reported during early spring. These factors have been described as barriers for compliance with protective measures, whereas formal trainings and PPE observers reduced occupational infection.9,29-31 In terms of human resources management, even before the pandemic, a shortage of nurses and healthcare support workers forced a substantial proportion of HCWs to work overtime or in several departments or sites.32 This trend continued throughout the study period and most acutely in LTCFs, even if it was identified as a critical risk factor early in the pandemic.33 At the onset of the pandemic, given limited access to masks and insufficient COVID-19 epidemiological knowledge, PPE was recommended in Québec only for HCWs providing care to COVID-19 patients, but mask use for all patient and other coworker contacts was recommended only at the end of May 2020.34 Lack or insufficient access to PPE was reported by participants throughout the study period as the main reason for not wearing PPE during interactions with COVID-19 patients. Inadequate PPE and PPE shortages increased the risk of SARS-CoV-2 infection among HCWs, whereas consistently wearing a mask protected HCWs from infection in different settings in the United States, the United Kingdom, and China.19,35-37 Finally, frequent unprotected contacts with colleagues were reported during all the study periods, suggesting a lower perception of infection risk from coworkers than from patients. Interaction with SARS-CoV-2–infected coworkers increased risk of infection by 74% in a large German study.27 All of these risk factors appear to have decreased during the first pandemic wave but remained fairly common, even at the end of the study period.
Our study has several strengths and limitations. This case series is large and represents well the conditions of infected HCWs but not noninfected Québec HCWs. This study was adequate to identify occupations and settings most affected by COVID-19 in absolute terms, to identify important gaps in IPC measures, and to compare ACHs and LTCFs. Without a control group of noninfected HCWs, however, we were unable to estimate the impact of the various factors on the risk of COVID-19. Self-reporting of IPC practices, like hand hygiene and physical distancing, might suffer from desirability bias. Information about other important individual and organizational factors, such as ethnicity, type of training, testing strategies, human resources issues or work organization, was not collected. Despite these limitations, this study is one of the most complete published descriptions of the COVID-19 epidemic among HCWs in a large healthcare system and the evolution of IPC measures during the first pandemic wave.
In conclusion, Québec HCWs and their families were severely affected during the first wave of COVID-19. Lack of preparedness, gaps in IPC, and a low perception of risk from contacts with coworkers likely contributed to their infection. HCW safety will require broader occupational health and safety approaches using the hierarchy of preventive measures and involving both HCW representatives and managers.
Acknowledgments
We thank Jo-Anne Costa, Josiane Rivard, and Manale Ouakki as well as all interviewers who made this work possible.
Financial support
This study was funded by the Ministère de la santé et des services sociaux du Québec.
Conflicts of interest
G.D.S. received grant support from Pfizer. M.D. received grant support from Pfizer, GSK, and Medicago. All others authors report no conflicts of interest relevant to this article.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.160.
click here to view supplementary material
References
- 1. Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in healthcare workers—a systematic review and meta-analysis. Am J Emerg Med 2020;38:1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recensement national des cas de COVID-19 chez les professionnels en établissements de santé. Mise à jour le 30 juin 2020. Santé Publique France website. https://www.santepubliquefrance.fr/etudes-et-enquetes/recensement-national-des-cas-de-covid-19-chez-les-professionnels-en-etablissements-de-sante. Published 2020. Accessed October 30, 2020.
- 3.Análisis de los casos de COVID-19 en personal sanitario notificados a la Red Nacional de Vigilancia Epidemiológica hasta el 10 de mayo en España. Fecha del informe: 29-05-2020. Ministerio de Sanidad de España website. Éhttps://www.ÉPIcentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_30-giugno-2020.pdf. Published 2020. Accessed October 30, 2020.
- 4.Épidemia COVID-19 Aggiornamento nazionale 30 giugno 2020 – ore 11:00. Instituto Superior di Sanità website. Éhttps://www.ÉPIcentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_30-giugno-2020.pdf. Published 2020. Accessed October 30, 2020.
- 5. Schwartz KL, Achonu C, Buchan SA, et al. Epidemiology, clinical characteristics, household transmission, and lethality of severe acute respiratory syndrome coronavirus-2 infection among healthcare workers in Ontario, Canada. PLoS One 2020;15:e0244477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus disease 2019 (COVID-19): epidemiology update. Government of Canada website. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html#a3. Published 2020. Accessed February 2, 2021.
- 7. Harrison D, Muradali K, El Sahly H, Bozkurt B, Jneid H. Impact of the SARS-CoV-2 pandemic on healthcare workers. Hosp Pract 2020;48:161–164. [DOI] [PubMed] [Google Scholar]
- 8. Chen M, Wei X, Wang Z. Protecting healthcare workers from SARS-CoV-2 and other infections. Epidemiol Infect 2020;148:e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lentz RJ, Colt H, Chen H, et al. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: the global ACT-HCP case-control study. Infect Control Hosp Epidemiol 2020. doi: 10.1017/ice.2020.455. [DOI] [PMC free article] [PubMed]
- 10. Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife 2020;9:e60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng C, Hafezi N, Cooper V, et al. Characteristics and transmission dynamics of COVID-19 in healthcare workers at a London Teaching Hospital. J Hosp Infect 2020;106:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkins J, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in healthcare workers in Chicago. medRxiv 2020. doi: 10.1101/2020.09.11.20192385. [DOI] [PMC free article] [PubMed]
- 13. Gómez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2020. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed]
- 14. Hughes MM, Groenewold MR, Lessem SE, et al. Update: characteristics of healthcare personnel with COVID-19 — United States, February 12–July 16, 2020. Morb Mortal Wkly Rep 2020;69:1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Data tables 2016 Census. Québec. Statistics Canada website. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/index-eng.cfm. Published 2016. Accessed October 30, 2020.
- 16.Statistiques. Médecins actifs seulement. Collège des Médecins du Québec website. http://www.cmq.org/statistiques/generalites.aspx. Published 2020. Accessed October 30, 2020.
- 17.Rapport statistique sur l’effectif infirmier, 2018-2019. Le Québec et ses régions. Ordre des infirmières et infirmiers du Québec website. https://www.oiiq.org/documents/20147/3410233/Rapport_statistique_2018-2019.pdf. Published 2019. Accessed October 30, 2020.
- 18.Rapport annuel, 2018-2019. Ordre des infirmières et infirmiers auxiliaires du Québec website. https://www.oiiaq.org/publications/rapports-annuels. Published 2019. Accessed October 30, 2020.
- 19. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. Lancet 2020;5:e475–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abo-Leyah H, Gallant S, Cassidy D, et al. Seroprevalence of SARS-COV-2 antibodies in Scottish healthcare workers. medRxiv 2020. doi: 10.1101/2020.10.02.20205641. [DOI] [PMC free article] [PubMed]
- 21. Wei J-T, Liu Z-D, Fan Z-W, Zhao L, Cao W-C. Epidemiology of and risk factors for COVID-19 infection among healthcare workers: a multicentre comparative study. Int J Environ Res Public Health 2020;17:7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galan I, Velasco M, Casas ML, et al. SARS-CoV-2 seroprevalence among all workers in a teaching hospital in Spain: unmasking the risk. medRxiv 2020. doi: 10.1101/2020.05.29.20116731. [DOI]
- 23. Jespersen S, Mikkelsen S, Greve T, et al. Severe acute respiratory syndrome coronavirus 2 seroprevalence survey among 17,971 healthcare and administrative personnel at hospitals, prehospital services, and specialist practitioners in the central Denmark region. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1471. [DOI] [PMC free article] [PubMed]
- 24. Akinbami LJ, Vuong N, Petersen LR, et al. SARS-CoV-2 Seroprevalence among healthcare, first response, and public safety personnel, Detroit metropolitan area, Michigan, USA, May–June 2020. Emerg Infect Dis 2020; 26:2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blairon L, Mokrane S, Wilmet A, et al. Large-scale, molecular and serological SARS-CoV-2 screening of healthcare workers in a 4-site public hospital in Belgium after COVID-19 outbreak. J Infect 2020. doi: 10.1016/j.jinf.2020.07.033. [DOI] [PMC free article] [PubMed]
- 26. Rudberg A-S, Havervall S, Månberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun 2020;11:5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erber J, Kappler V, Haller B, et al. Strategies for infection control and prevalence of anti–SARS-CoV-2 IgG in 4,554 employees of a university hospital in Munich, Germany. medRxiv 2020. doi: 10.1101/2020.10.04.20206136. [DOI] [PMC free article] [PubMed]
- 28. Dimcheff DE, Schildhouse RJ, Hausman MS, et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans’ Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol 2020. doi: 10.1017/ice.2020.1220. [DOI] [PMC free article] [PubMed]
- 29. Zhou F, Li J, Lu M, et al. Tracing asymptomatic SARS-CoV-2 carriers among 3,674 hospital staff:a cross-sectional survey. EClinicalMedicine 2020;26:100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Houghton C, Meskell P, Delaney H, et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev 2020;4:CD013582. [DOI] [PMC free article] [PubMed]
- 31. Savoia E, Argentini G, Gori D, Neri E, Piltch-Loeb R, Fantini MP. Factors associated with access and use of PPE during COVID-19: a cross-sectional study of Italian physicians. PLoS One 2020;15:e0239024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rinfret M. Rapport annuel 2019–2020. Protecteur du citoyen website. https://rapportannuel.protecteurducitoyen.qc.ca/. Published 2020. Accessed October 30, 2020.
- 33. Sharma M, Creutzfeldt CJ, Lewis A, et al. Healthcare professionals’ perceptions of critical care resource availability and factors associated with mental well-being during COVID-19: results from a US survey. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa13111. [DOI] [PMC free article] [PubMed]
- 34.Directives cliniques aux professionnels et au réseau pour la COVID-19. Ministère de la Santé et des Services Sociaux Québec website. https://www.msss.gouv.qc.ca/professionnels/covid-19/directives-cliniques-aux-professionnels-et-au-reseau/prevention-et-controle-des-infections/. Published 2020. Accessed October 30, 2020.
- 35. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel in a multistate hospital network—13 academic medical centers, April–June 2020. Morb Mortal Wkly Rep 2020;69:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daga S, Jafferbhoy S, Menon G, et al. Does gender or religion contribute to the risk of COVID-19 in hospital doctors? medRxiv 2020. doi: 10.1101/2020.06.15.20125450. [DOI]
- 37. Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020;71:2218–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.160.
click here to view supplementary material