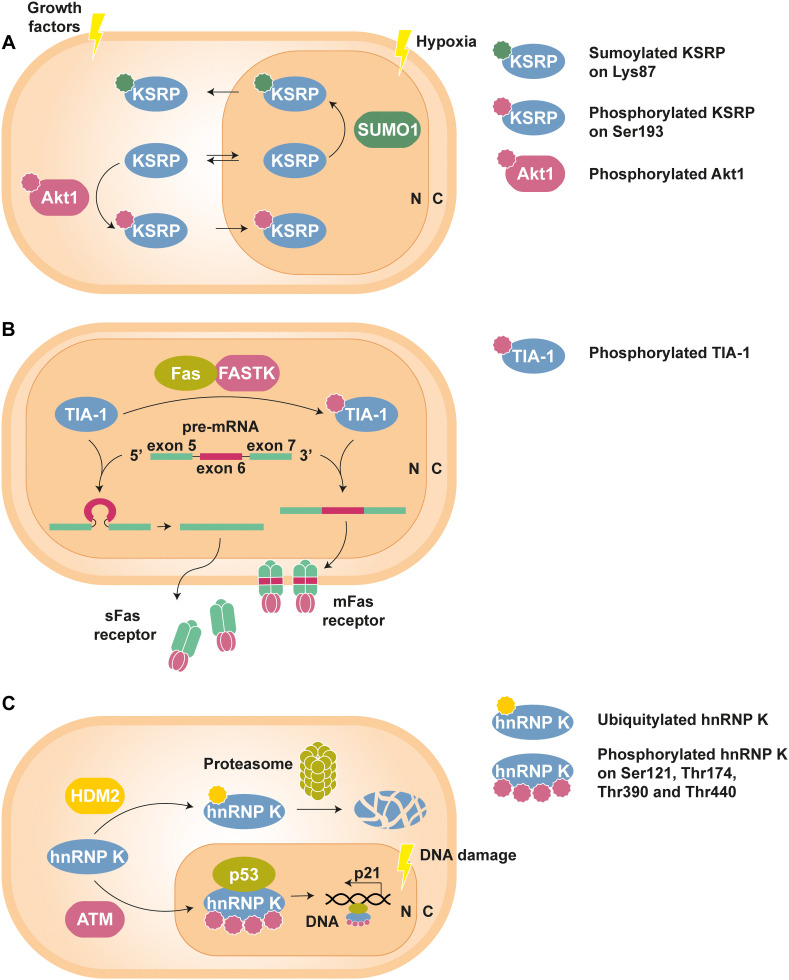
FIGURE 1.
Examples of PTM-mediated regulation of RBPs. (A) KSRP can shuttle between nucleus (N) and cytoplasm (C) to perform specific functions in each compartment. However, phosphorylation at Ser193 by Akt1, stimulated by growth factors, promotes the translocation of KSRP to the nucleus, whereas hypoxia-induced SUMOylation at Lys83 leads to its nuclear export (Díaz-Moreno et al., 2009; Yuan et al., 2017). (B) Phosphorylation of TIA-1 by FASTK improves its ability to recruit the U1 snRNP spliceosomal complex to the 5′ splice site region of the Fas receptor pre-mRNA exon 6. The resulting mature mRNA will express mFas, which plays an important role in the extrinsic apoptosis signaling pathways. In contrast, splicing of Fas receptor in the presence of unphosphorylated TIA-1 results in exon 6 skipping and the synthesis of sFas, that blocks apoptosis (Förch et al., 2002; Izquierdo and Valcárcel, 2007). (C) Under standard conditions, hnRNP K is targeted by the E3 Ub-ligase HDM2 for proteasomal degradation. Nonetheless, DNA damage triggers ATM-dependent phosphorylation of hnRNP K at Ser121, Thr174, Thr390, and Thr440, thus lowering its turnover rate. In addition, phosphorylated hnRNP K stimulates p53-mediated p21 gene expression, which causes cell cycle arrest (Moumen et al., 2005, 2013).