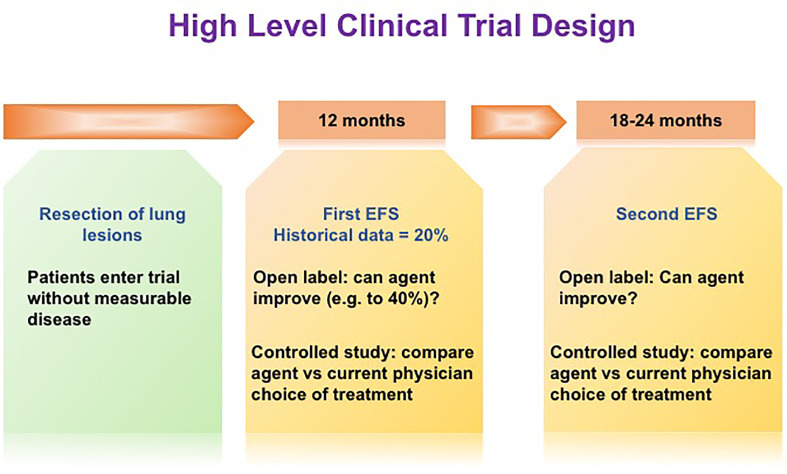
Figure 3.
High level clinical trial design. In order to increase the ability to observe clinical impacts of novel dormant DTCs-targeting drugs, novel trial endpoints may be required. We propose an innovative trial design, namely, improving EFS at a later time point, e.g. 6-12 months following the initial EFS at 12 months post-surgery.