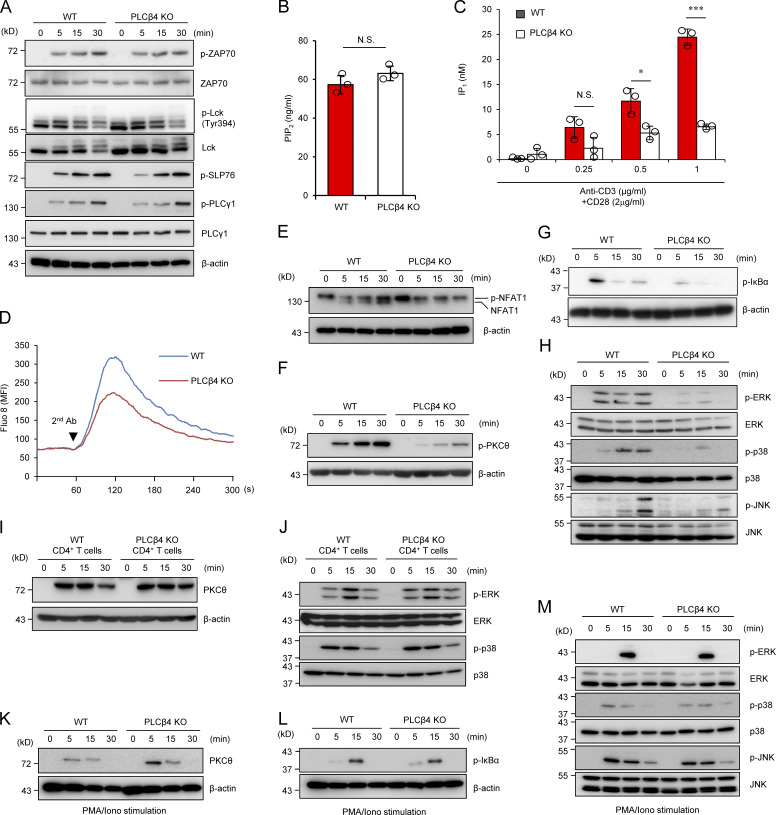
Figure 3.
PLCβ4-deficient CD8+ T cells display impaired activation of signaling cascades downstream of IP3. (A) Purified CD8+ T cells from WT or PLCβ4-deficient mice were stimulated with anti-CD3 (5 µg) and anti-CD28 (2 µg/ml) for indicated times. Indicated proteins were detected by Western blotting using specific phospho- or nonphospho Abs. (B) Concentrations of PIP2 in the lysates of purified CD8+ T cells from WT and PLCβ4-deficient mice were measured by ELISA. (C) Purified CD8+ T cells from WT and PLCβ4-deficient mice were stimulated with indicated doses of anti-CD3 and anti-CD28 for 1 h and lysed. IP1 levels were determined by ELISA. (D) Purified CD8+ T cells from WT or PLCβ4-deficient mice were loaded with Fluo-8 AM and stimulated with anti-CD3 (1 µg), followed by cross-linking (arrowhead). Ca2+ flux was measured by flow cytometry. The x axis shows real-time Ca2+ release followed for 300 s, and the y axis shows the intensity of the increase in intracellular Ca2+ concentration. MFI, mean fluorescence intensity. (E–H) Purified CD8+ T cells from WT or PLCβ4-deficient mice were stimulated with anti-CD3 (5 µg) and anti-CD28 (2 µg/ml) for indicated times. (E) Dephosphorylation of NFAT1 was detected by Western blotting with ant-NFAT1. (F and G) The phosphorylation of PKCθ (F) and IκBα (G) was determined by Western blotting with indicated Abs. (H) Activation of ERK, p38, and JNK was detected by Western blotting using specific phospho- or nonphospho Abs. (I and J) Purified CD4+ T cells from WT or PLCβ4-deficient mice were stimulated with anti-CD3 (5 µg) and anti-CD28 (2 µg/ml) for various times. (I) The phosphorylation of PKCθ was determined by Western blotting with indicated Abs. (J) Activation of ERK and p38 was detected by Western blotting using specific phospho Abs. (K–M) Purified CD8+ T cells from WT or PLCβ4-deficient mice were stimulated with PMA (50 ng/ml) and ionophore (1 µg/ml) for indicated times. The phosphorylation of PKCθ (K), IκBα (L), and MAP kinases such as ERK, p38, and JNK (M) were detected by Western blotting using specific phospho Abs. Indicated values are means ± SD of three biological replicates (B and C). *, P < 0.05; ***, P < 0.001, N.S., nonsignificant. Data are representative of three (A and D–H) or two (I–M) independent experiments.