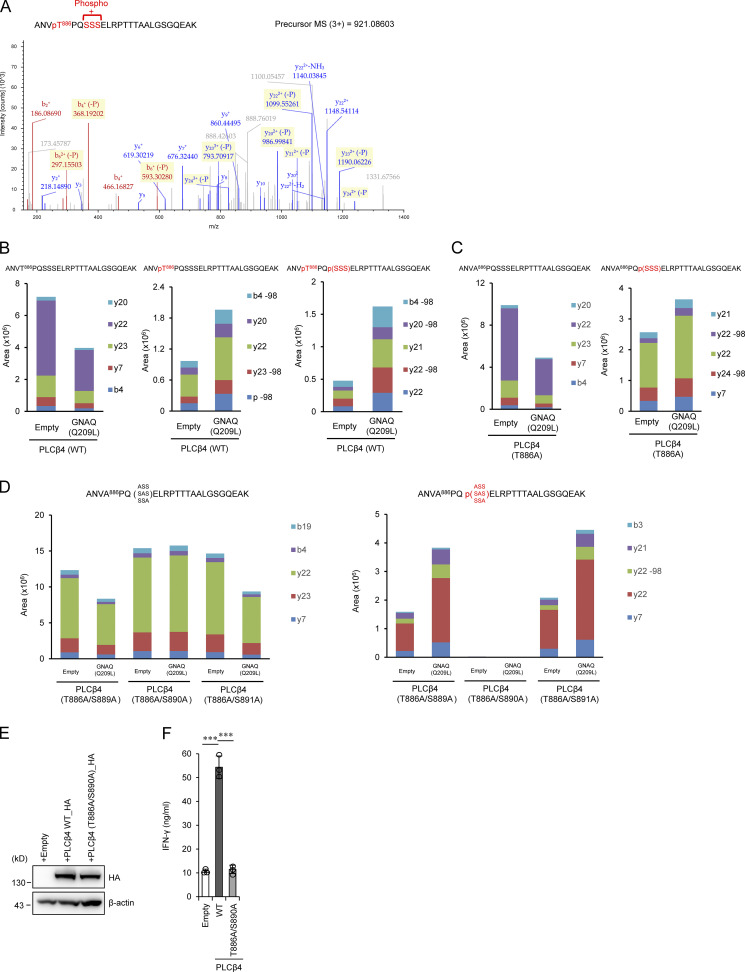
Figure 6.
GNAQ-induced PLCβ4 phosphorylation is important for CD8+ T cell activation. (A) 293T cells transiently cotransfected with HA-tagged WT PLCβ4 and Flag-tagged GNAQ (Q209L) were subjected to immunoprecipitation with anti-HA antibody followed by tryptic digestion and LC-MS/MS analysis. The MS/MS spectrum suggested that phosphorylation of PLCβ4 occurs at T886 and S889, S890, or S891. (B–D) 293T cells transiently cotransfected with HA-tagged WT (B), T886A (C), or T886A/S889A, T886A/S890A, and T886A/S891A PLCβ4 (D) and Flag-tagged GNAQ (Q209L) or the empty vector were subjected to immunoprecipitation with anti-HA antibody followed by tryptic digestion. Phosphopeptides and nonphosphopeptides of the A883-K908 peptides were quantified by targeted MS using the parallel reaction monitoring method. It should be noted that the phosphopeptide was completely eliminated by T886A/S890A mutation. (E) Purified CD8+ T cells from PLCβ4-deficient mice were transduced with empty, PLCβ4 (WT), or PLCβ4 (T886A/S890A) construct. Protein levels of PLCβ4 (WT) and mutant of PLCβ4 (T886A/S890A) in the indicated lysates were determined by Western blotting with indicated Abs. (F) Purified CD8+ T cells from PLCβ4-deficient mice were retrovirally transduced with empty, PLCβ4 (WT), or mutant of PLCβ4 (T886A/S890A) construct. The transduced cells were stimulated with anti-CD3 (0.5 µg) and anti-CD28 (1 µg/ml) for 72 h. Concentrations of IFN-γ in the culture supernatants were measured by ELISA. Indicated values are means ± SD of three biological replicates (F). ***, P < 0.001. Data are representative of two independent experiments (A–E).