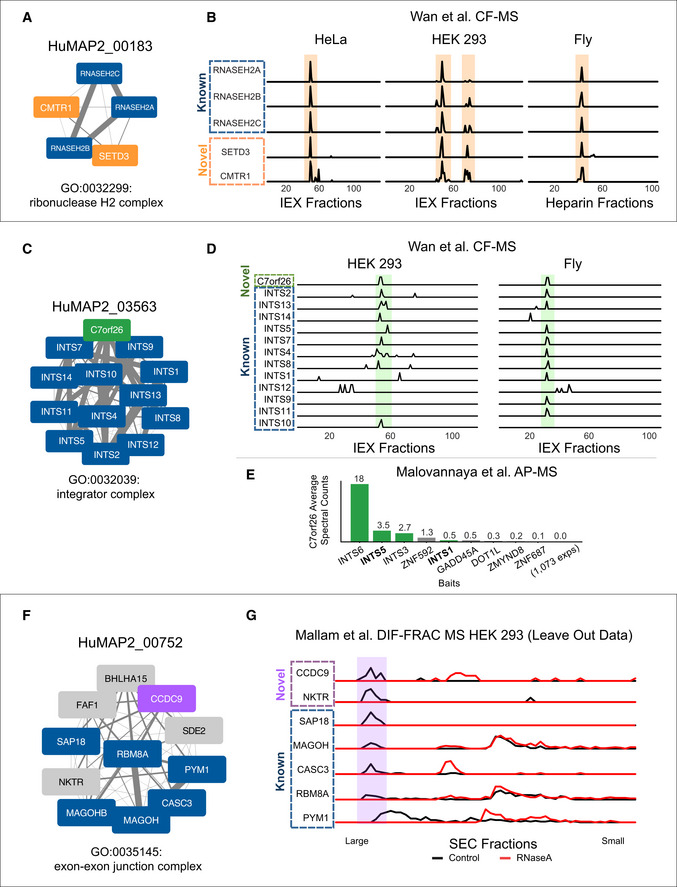
SETD3 and CMTR1 are identified as co‐complex interactors with the Ribonuclease H2 complex which provides a possible mechanistic explanation for their role in viral infection.
Sparkline elution profiles from multiple orthogonal co‐fractionation experiments demonstrate a strong degree of co‐elution among subunits in the SETD3‐CMTR1‐RNAse H2 complex. Weight of network edges represents confidence of interactions. X‐axis represents fraction collected along biochemical separation. Y‐axis for each row represents observed protein abundance.
The uncharacterized protein, C7orf26, is identified as part of the Integrator complex.
Sparkline elution profiles show a high degree of correlation between C7orf26 and subunits of the Integrator complex from multiple orthogonal co‐fractionation experiments.
The association of C7orf26 and Integrator complex is additionally supported by affinity purification mass spectrometry (AP‐MS) experiment where C7orf26 is pulled down with Integrator subunit baits.
The uncharacterized protein, CCDC9, is identified as co‐complex with the exon–exon junction complex (EJC), a ribonucleoprotein complex involved in splicing.
Sparkline elution profiles from the independently collected RNA DIF‐FRAC size exclusion chromatography (SEC) experiment show CCDC9 co‐elutes with known subunits of the EJC when RNA is present (black). The elution profiles also show CCDC9 is sensitive to RNAse A treatment (shift of elution peak between black and red profiles) as are the subunits of the EJC further supporting CCDC9's participation in this known ribonucleoprotein complex.