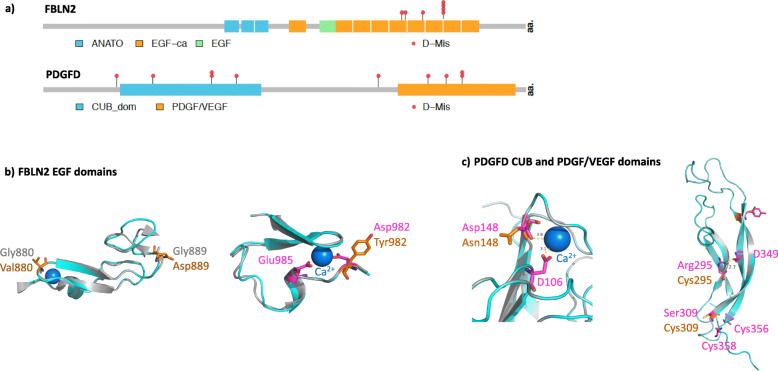
Fig. 2.
Locations of PAH-associated rare variants within FBLN2 and PDGFD protein structures. a Variants and conserved domains within two-dimensional protein structures. The numbers of variants at each amino acid position is indicated along the y-axes. D-MIS, predicted deleterious missense; LGD, likely gene-disrupting (stopgain, frameshift, splicing). FBLN2: ANATO, anaphylatoxin-like 2; EGF-ca, calcium-binding endothelial growth factor-like 1; EGF, non-calcium-binding EGF domain. PDGFD: CUB, complement subcomponent; PDGF/VEGF, platelet-derived growth factor/vascular endothelial-derived growth factor domain. b FBLN2 residues 858-900: p.(Gly880Val) and p.(Gly889Asp) change the conserved i+2 glycine residues of type II reverse turns within an EGF domain. Residues 981-1011: recurrent p.(Asp982Tyr) changes a residue within the highly conserved DXXE motif/calcium-binding site within an EGF domain. c PDGFD residues 43-180: p.(Asp148Asn) predicted to destroy the Ca++ binding site of the CUB domain. Residues 264-364: p.(Arg295Cys) disrupts a hydrogen bond and p.(Ser309Cys) may create a new disulfide bond in the PDGF/VEGF domain