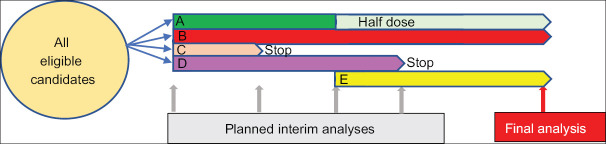
Figure 4.
Adaptive trial design. All eligible patients are randomized to Placebo (p) or treatment Groups A, B, C or D. Interim analysis is run at defined time points and any treatment arm with low probability of benefit or potential harm is subjected to dose modification accordingly. A newly discovered treatment (e) can be added at later stage if required