Abstract
Left ventricular hypertrophy refers to a pathologic increase in left ventricular mass and is associated with an increased risk of subsequent cardiovascular morbidity and mortality from any cause. In the development of left ventricular hypertrophy there is growth of cardiomyocytes and accumulation of extracellular matrix and fibrosis. The actions are partly induced by angiotensin II, the principal effector of the renin‐angiotensin‐aldosterone system, binding to the AT1 receptor. Biochemical markers, some implicated in inflammatory changes, correlate with changes in left ventricular mass. The reduction in left ventricular mass brought about with angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (ARB) therapy correlates with a reduction in these inflammatory changes, monitored by brain natriuretic peptide. Recent studies incorporating trials of ARBs have found ARBs to be more effective in reducing left ventricular mass than β blockers and possibly more effective than calcium antagonists. Initial studies suggest that ARBs and angiotensin‐converting enzyme inhibitors may have similar effects in terms of reducing left ventricular hypertrophy, and the combination of angiotensin‐converting enzyme inhibitors and ARBs is thought to be synergistic due to a more complete inhibition of the renin‐angiotensin‐aldosterone system. In conclusion, these agents are efficacious in antihypertensive therapy and can play an important role in the prevention or regression of left ventricular hypertrophy due to hypertension.
Left ventricular hypertrophy (LVH) refers to a pathologic increase in left ventricular mass (LVM) and can be a manifestation of preclinical cardiovascular disease. LVH is associated with an increased risk of subsequent cardiovascular morbidity and mortality from any cause.
LVH occurs in response to pressure overload (such as that imposed by hypertension) or volume overload (such as that imposed by aortic regurgitation or some form of renal failure).1, 2, 3 With pressure overload, LVH occurs primarily through hypertrophy of existing cardiomyocytes (i.e., an increase in existing cell size but not in cell number). As a result, hypertensive LVH often presents as an increase in relative wall thickness to compensate for the increased blood pressure (BP). This parallel addition of sarcomeres (segment of myofibril representing the functional unit of heart muscle) produces an increase in sarcomere width resulting in increased wall thickness‐to‐lumen volume ratio. This is referred to as concentric hypertrophy.
With volume overload, the resultant LVH enables a high stroke volume to be sustained. In this case, dilation leads to an increase in LVM with no increase in relative wall thickness. This type of hypertrophy occurs primarily via cardiomyocyte elongation and addition of sarcomeres at the ends of muscle fibers in series, to produce an increase in ventricular volume and a normal‐to‐decreased wall thickness‐to‐lumen volume ratio. This is known as eccentric hypertrophy.
Of the two types of LVH, the concentric hypertrophy pathogenesis is triggered by hypertension and, therefore, it would be logical to assume that this may be the type of LVH that responds best to antihypertensive therapy. However, eccentric hypertrophy is not an uncommon consequence of hypertension, and both types of hypertrophy have been shown to respond to certain types of antihypertensive therapy independent of BP lowering.4
LVH is characterized by increases in the amount of surrounding connective tissue, with cardiac fibrosis involving increased deposition of collagen.5 Over time, the thickened fibers of the hypertrophied heart become less able to contract and relax, and changes in collagen result in increased stiffness. In addition to the mechanical stress of pressure overload, various systemic and locally expressed factors, such as cytokine transforming growth factor‐β1 and angiotensin II, play a role in the development of LVH associated with hypertension. Increased expression of fetal genes, including genes for natriuretic peptides and fetal contractile proteins, is also observed during cardiac hypertrophy.6
The most common methods of detection of LVH are the electrocardiogram and echocardiography, with echocardiography having a higher sensitivity for LVH than electrocardiogram.2 In echocardiography, left ventricular (LV) dimensions are used to calculate LVM by a validated formula, which correlates closely to LVM at autopsy (r=0.90; p<0.001). LVH can be defined as LVM indexed to body surface area >116 g/m2 in men and >104 g/m2 in women,7 with some variations between different studies.8, 9 An electrocardiogram has relatively low sensitivity but high specificity.8, 10 The product of QRS duration times Cornell voltage (with adjustment of 6 mm in women and a partition value of >2440 mm × ms) is recommended to recognize LVH and yields the best sensitivity.8, 10 LVH is an independent predictor of adverse cardiovascular outcomes.8, 11, 12, 13, 14 Among patients with hypertension, presence of LVH increases the risk of progression to heart failure.15
IMPORTANCE OF ANGIOTENSIN II IN LVH
Angiotensin II is the principal effector of the renin‐angiotensin‐aldosterone system. The effects of angiotensin II are mediated by its binding to two subtypes of cell surface receptors, AT1 and AT2. Binding of angiotensin II to the AT1 receptor mediates its classical physiologic actions: regulation of BP and regulation of plasma volume.16 Many of the responses to angiotensin II mediated through the AT1 receptor can be deleterious, e.g., sympathetic nervous system activation‐resulting in reinforced vasoconstriction and increased rate and force of heart contraction, renal tubular sodium reabsorption, and decreased renal blood flow.16, 17
In addition, angiotensin II, via its interaction with the AT1 receptor, is a potent promoter of cardiomyocyte growth and is involved in the development of LVH (Figure). Angiotensin II‐mediated effects on cardiovascular cell growth may take place through a variety of mechanisms. Stimulation of early response genes by angiotensin II via the AT1 receptor results in increased production of growth factors, vasoconstrictor agents, adhesion molecules, integrins, and tumor necrosis factor α.16 Angiotensin II, via the AT1 receptor, also induces the accumulation of extracellular matrix and fibrosis,16, 18, 19, 20 and it may exert its effects on remodeling and fibrosis by stimulating aldosterone.5 It is thought that binding of angiotensin II to the AT2 receptor mediates generally favorable effects that counteract the responses induced by the AT1 receptor.21 However, this requires further clarification.22, 23, 24
Figure.
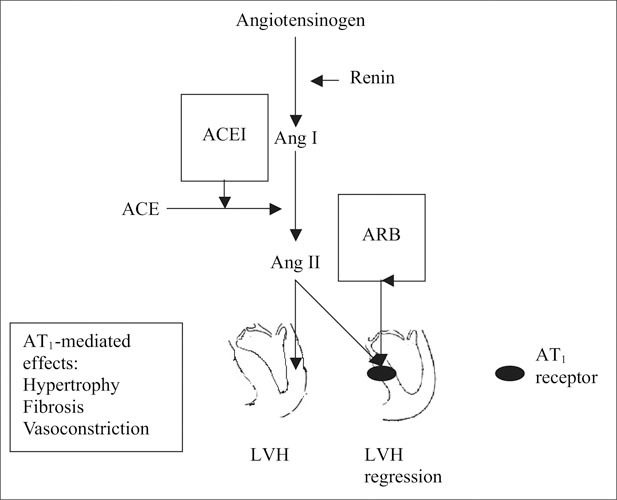
The scheme shows inhibition of the renin‐angiotensin system with an angiotensin‐converting enzyme inhibitor (ACEI) and an angiotensin receptor blocker (ARB). Blocking the effects of angiotensin (Ang) II induces regression of left ventricular hypertrophy (LVH).
POTENTIAL BIOCHEMICAL MARKERS OF LVH AND EFFECT OF AT1 RECEPTOR BLOCKADE
Several biochemical markers have been associated with LVH. Many of these have been implicated in the inflammatory changes resulting from hypertension, such as monocyte chemoattractant protein‐1, which controls monocyte function through its receptor CCR2,25 and plasma B‐type natriuretic peptide, which is an independent marker of increased LVM.26, 27 AT1 receptor blockade may reduce such LVH markers: Suppression of monocyte chemoattractant protein‐1 expression induced by an angiotensin II receptor blocker (ARB) and other agents that block the renin‐angiotensin system results in reduced monocyte/macrophage accumulation and may, in part, account for the BP‐independent regression of LVH.25, 28, 29 More research of the importance of inflammatory markers for LVH regression in humans is needed, however. The reduction in LVM brought about by ARB therapy correlates with a reduction in B‐type natriuretic peptide.30
EFFECTS OF ANTIHYPERTENSIVE AGENTS ON LVH
Most antihypertensive therapy can prevent progression and even achieve partial regression or reversal of LVH in terms of reduction in LVM.31 However, the different antihypertensive agents vary in their mode of action and in their propensity to decrease LVM independent of BP lowering; thus, it is possible that some will be more effective than others in regression of concentric and eccentric LVH. In 1992, a meta‐analysis of 109 studies of antihypertensive therapy (that did not include ARBs) in LVH patients showed that angiotensin‐converting enzyme (ACE) inhibitors, β blockers, calcium antagonists, and diuretics all reduced LVM.32 Diuretics predominantly reduced ventricular diameter, whereas the other agents predominantly reduced wall thickness. The LVM‐reducing effect was most marked with ACE inhibitors in this review.32
Other early meta‐analyses indicated that ACE inhibitors and calcium antagonists provided greater improvements in LVH parameters than did β blockers and other antihypertensive medications.32, 33, 34, 35 Concentric changes are seen more in women and eccentric changes more in men36—this may, in part, explain the gender‐specific effects of some but not all antihypertensive agents.37 In fact, Safar and Smulyan37 suggested that the development of hypertension and structural changes in the heart and blood vessels over time may be quite different in men and women and explain why some trial evidence showed benefit of ACE inhibitor treatment in men but not in women.
MECHANISM OF ACTIONS OF ARBs
ARBs are selective inhibitors of the AT1 receptors; the mechanism of action thus differs from that of other antihypertensives, including the mechanism of action of ACE inhibitors. Alternate pathways exist for production of angiotensin II beyond its conversion from angiotensin I by ACE.21 Therefore, selective blockade of the AT1 receptor by ARBs would intuitively seem to provide the most effective means of combating the deleterious effects of angiotensin II in processes such as LVH (Figure).
COMPARISON OF ARBs WITH OTHER ANTIHYPERTENSIVE THERAPY ON LVH
There may be differential effects of ARBs compared with ACE inhibitors, calcium antagonists, and β blockers on reducing LVH. A recent meta‐analysis incorporating studies of ARBs found that ARBs are effective in reducing LVM and more effective than β blockers.38 In the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study,39 ARB‐based antihypertensive therapy with losartan achieved a significantly greater reduction in LVH than did β blocker‐based therapy with an atenolol‐based regimen, despite similar reductions in BP. Blocking the effects of angiotensin II on the AT1 receptor is thus likely to achieve greater LVH regression than therapy with some other agents with different antihypertensive mechanisms, and this may help explain the LIFE study findings. Similar results were seen in the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study,40 in which the ARB irbesartan achieved a greater reduction in LVM than did a β blocker atenolol‐based regimen. Studies with ACE inhibitors also report the same.38 It should be remembered that all these studies, including LIFE, were multiple drug studies, usually with a diuretic as part of the therapeutic program.
Studies to date also suggest that ARBs may be more effective than calcium antagonists in reducing LVM41, 42, 43; however, this may require further investigation. As noted, initial studies suggest that ARBs and ACE inhibitors may produce similar degrees of LVM reduction,9, 44 and that combined ACE inhibition and AT1 receptor blockade may be more effective than either monotherapy in reducing LVM.45, 46, 47 The combined effect of AT1 receptor blockade and ACE inhibition is thought to be synergistic (at optimal low doses), and the increase in LVH regression seen with combined therapy compared with monotherapy is thought to be due to a more complete inhibition of the renin‐angiotensin‐aldosterone system, possibly involving interactions with sympathetic kinases and kinins.46 The significant correlation between plasma aldosterone levels and LVM in hypertensive patients with concentric LVH48 suggest that the marked reduction in LVM seen with combined AT1 receptor blockade and ACE inhibition may, in part, be linked to the reduction in plasma aldosterone noted with these agents.25, 28, 29 Thus, combination of an aldosterone antagonist with an ACE inhibitor or ARB may prove to be even better in lowering BP and reducing proteinuria and LVH, but there are no data available to confirm this.
EFFECTS OF IMPROVING LVH ON CARDIOVASCULAR OUTCOMES
Improvements in LVH (reduced LVM) with antihypertensive therapy usually correlate with improvements in long‐term cardiovascular outcomes, possibly independent of BP lowering7, 59, 53; however, in one study that included an ACE inhibitor,51 a difference in BP may have played a role in outcome. In the LIFE study, an ARB‐based regimen prevented strokes better than therapy based on a β blockerβwith similar reductions in BP.54
CONCLUSIONS
LVH predicts a poor prognosis independent of the patient's BP level. Several major studies have presented evidence suggesting that LVH should be a therapeutic target in addition to BP control. The actions of angiotensin II, mediated through the AT1 receptor, are critical in the development of LVH. Therapy interfering with the interaction between angiotensin II and the AT1 receptor regresses LVH in patients with hypertension. BP control should be the first priority in reducing LVH. ARBs are efficacious in lowering BP and are recommended as possible initial therapy for hypertension and, like other agents that affect the renin‐angiotensin system, may confer LVH reduction and cardiovascular benefits beyond BP lowering. Medications that block the renin‐angiotensin‐aldosterone system, including ARBs, may prove to be agents of choice in hypertensive patients to prevent LVH and produce regression of LVH when present. It should be remembered that all of the trials that have suggested this were multidrug trials that included an ACE inhibitor or ARB, usually with a diuretic or other medication.
Disclosure: This project was supported by Daiichi Sankyo, Inc., Parsippany, NJ.
References
- 1. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. [DOI] [PubMed] [Google Scholar]
- 3. Diez J, Gonzalez A, Lopez B, et al. Effects of antihypertensive agents on the left ventricle: clinical implications. Am J Cardiovasc Drugs. 2001;1:263–279. [DOI] [PubMed] [Google Scholar]
- 4. Bleumink GS, Deinum J, Mosterd A, et al. Antihypertensive treatment is associated with improved left ventricular geometry: the Rotterdam Study. Pharmacoepidemiol Drug Saf. 2004;13:703–709. [DOI] [PubMed] [Google Scholar]
- 5. Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–272. [DOI] [PubMed] [Google Scholar]
- 6. Hannan RD, Jenkins A, Jenkins AK, et al. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol. 2003;30:517–527. [DOI] [PubMed] [Google Scholar]
- 7. Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 8. Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. [DOI] [PubMed] [Google Scholar]
- 9. Cuspidi C, Muiesan ML, Valagussa L, et al. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens. 2002;20:2293–2300. [DOI] [PubMed] [Google Scholar]
- 10. Okin PM, Roman MJ, Devereux RB, et al. Time‐voltage QRS area of the 12‐lead electrocardiogram: detection of left ventricular hypertrophy. Hypertension. 1998;31:937–942. [DOI] [PubMed] [Google Scholar]
- 11. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 12. Lonn E, Mathew J, Pogue J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high‐risk patients. Eur J Cardiovasc Prev Rehabil. 2003;10:420–428. [DOI] [PubMed] [Google Scholar]
- 13. Ciardullo AV, Azzolini L, Bevini M, et al. A diagnosis of left ventricular hypertrophy on ECG is associated with a high cardiovascular risk: findings from a 40‐to 69‐year‐old cohort in general practice. Fam Pract. 2004;21:63–65. [DOI] [PubMed] [Google Scholar]
- 14. Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. [DOI] [PubMed] [Google Scholar]
- 15. Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 16. Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. [DOI] [PubMed] [Google Scholar]
- 17. Unger T. Neurohormonal modulation in cardiovascular disease. Am Heart J. 2000;139:S2–S8. [DOI] [PubMed] [Google Scholar]
- 18. Booz GW, Baker KM. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res. 1995;30:537–543. [PubMed] [Google Scholar]
- 19. Gonzalez A, Lopez B, Querejeta R, et al. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol. 2002;34:1585–1593. [DOI] [PubMed] [Google Scholar]
- 20. Mascareno E, Siddiqui MA. The role of Jak/STAT signalling in heart tissue renin‐angiotensin system. Mol Cell Biochem. 2000;212:171–175. [PubMed] [Google Scholar]
- 21. Unger T. The role of the renin‐angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89(suppl):3A–10A. [DOI] [PubMed] [Google Scholar]
- 22. Ichihara S, Senbonmatsu T, Price E Jr, et al. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II‐induced hypertension. Circulation. 2001;104:346–351. [DOI] [PubMed] [Google Scholar]
- 23. Diez J. Angiotensin II and the hypertensive heart: a role for the AT2 receptor? J Hypertens. 2004;22:879–882. [DOI] [PubMed] [Google Scholar]
- 24. Gross V, Obst M, Kiss E, et al. Cardiac hypertrophy and fibrosis in chronic L‐NAME‐treated AT2 receptor‐deficient mice. J Hypertens. 2004;22:997–1005. [DOI] [PubMed] [Google Scholar]
- 25. Ishibashi M, Hiasa K, Zhao Q, et al. Critical role of monocyte chemoattractant protein‐1 receptor CCR2 on monocytes in hypertension‐induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. [DOI] [PubMed] [Google Scholar]
- 26. Almeida P, Azevedo A, Rodrigues R, et al. B‐type natriuretic peptide and left ventricular hypertrophy in hypertensive patients. Rev Port Cardiol. 2003;22:327–336. [PubMed] [Google Scholar]
- 27. Uusimaa P, Tokola H, Ylitalo A, et al. Plasma B‐type natriuretic peptide reflects left ventricular hypertrophy and diastolic function in hypertension. Int J Cardiol. 2004;97:251–256. [DOI] [PubMed] [Google Scholar]
- 28. Behr TM, Willette RN, Coatney RW, et al. Eprosartan improves cardiac performance, reduces cardiac hypertrophy and mortality and downregulates myocardial monocyte chemoattractant protein‐1 and inflammation in hypertensive heart disease. J Hypertens. 2004;22:583–592. [DOI] [PubMed] [Google Scholar]
- 29. Tokuda K, Kai H, Kuwahara F, et al. Pressure‐independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension. 2004;43:499–503. [DOI] [PubMed] [Google Scholar]
- 30. Anan F, Takahashi N, Ooie T, et al. Candesartan, an angiotensin II receptor blocker, improves left ventricular hypertrophy and insulin resistance. Metabolism. 2004;53:777–781. [DOI] [PubMed] [Google Scholar]
- 31. Sheridan DJ. Regression of left ventricular hypertrophy: do antihypertensive classes differ? J Hypertens Suppl. 2000;18( suppl ):S21–S27. [PubMed] [Google Scholar]
- 32. Dahlöf B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studies. Am J Hypertens. 1992;5:95–110. [DOI] [PubMed] [Google Scholar]
- 33. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A metaanalysis of randomized double‐blind studies. JAMA. 1996;275:1507–1513. [PubMed] [Google Scholar]
- 34. Jennings G, Wong J. Regression of left ventricular hypertrophy in hypertension: changing patterns with successive meta‐analyses. J Hypertens Suppl. 1998;16(suppl 6);S29–S34. [PubMed] [Google Scholar]
- 35. Schmieder RE, Schlaich MP, Klingbeil AK, et al. Update on reversal of left ventricular hypertrophy in essential hypertension. (a meta‐analysis of all randomized double‐blind studies until December 1996). Nephrol Dial Transplant. 1998;13:564–569. [DOI] [PubMed] [Google Scholar]
- 36. Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. [DOI] [PubMed] [Google Scholar]
- 37. Safar ME, Smulyan H. Hypertension in women. Am J Hypertens. 2004;17:82–87. [DOI] [PubMed] [Google Scholar]
- 38. Klingbeil AU, Schneider M, Martus P, et al. A meta‐analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;l 15:41–46. [DOI] [PubMed] [Google Scholar]
- 39. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: The Losartan Intervention for Endpoint reduction in Hypertension (LIFE) Study. Circulation. 2003;108:684–690. [DOI] [PubMed] [Google Scholar]
- 40. Malmqvist K, Kahan T, Edner M, et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens. 2001;19:1167–1176. [DOI] [PubMed] [Google Scholar]
- 41. Martina B, Dieterle T, Weinbacher M, et al. Effects of losartan titrated to Losartan/Hydrochlorothiazide and amlodipine on left ventricular mass in patients with mild‐to‐moderate hypertension. A double‐blind randomized controlled study. Cardiology. 1999;92:110–114. [DOI] [PubMed] [Google Scholar]
- 42. Gaudio C, Ferri FM, Giovannini M, et al. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol. 2003;42:622–628. [DOI] [PubMed] [Google Scholar]
- 43. Yasunari K, Maeda K, Watanabe T, et al. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. J Am Coll Cardiol. 2004;43:2116–2123. [DOI] [PubMed] [Google Scholar]
- 44. De Rosa ML, Cardace P, Rossi M, et al. Comparative effects of chronic ACE inhibition and ATI receptor blocked losartan on cardiac hypertrophy and renal function in hypertensive patients. J Hum Hypertens. 2002;16:133–140. [DOI] [PubMed] [Google Scholar]
- 45. Avanza AC Jr, El Aouar LM, Mill JG. Reduction in left ventricular hypertrophy in hypertensive patients treated with enalapril, losartan or the combination of enalapril and losartan. Arq Bras Cardiol. 2000;74:103–117. [PubMed] [Google Scholar]
- 46. Raasch W, Johren O, Schwartz S, et al. Combined blockade of AT1‐receptors and ACE synergistically potentiates antihypertensive effects in SHR. J Hypertens. 2004;22:611–618. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki H, Kanno Y, Kaneko K, et al. Comparison of the effects of angiotensin receptor antagonist, angiotensin converting enzyme inhibitor, and their combination on regression of left ventricular hypertrophy of diabetes type 2 patients on recent onset hemodialysis therapy. Ther Apher Dial. 2004;8:320–327. [DOI] [PubMed] [Google Scholar]
- 48. Soylu A, Temizhan A, Duzenli MA, et al. The influence of aldosterone on the development of left ventricular geometry and hypertrophy in patients with essential hypertension. Jpn Heart]. 2004;45:807–821. [DOI] [PubMed] [Google Scholar]
- 59. Muiesan ML, Salvetti M, Rizzoni D, et al. Association of change in left ventricular mass with prognosis during long‐term antihypertensive treatment. J Hypertens. 1995;13:1091–1095. [DOI] [PubMed] [Google Scholar]
- 50. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. [DOI] [PubMed] [Google Scholar]
- 51. Mathew J, Sleight P, Lonn E, et al., for the Heart Outcomes Prevention Evaluation (HOPE). Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin‐converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. [DOI] [PubMed] [Google Scholar]
- 52. Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens. 2003;16:895–899. [DOI] [PubMed] [Google Scholar]
- 53. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 54. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]


