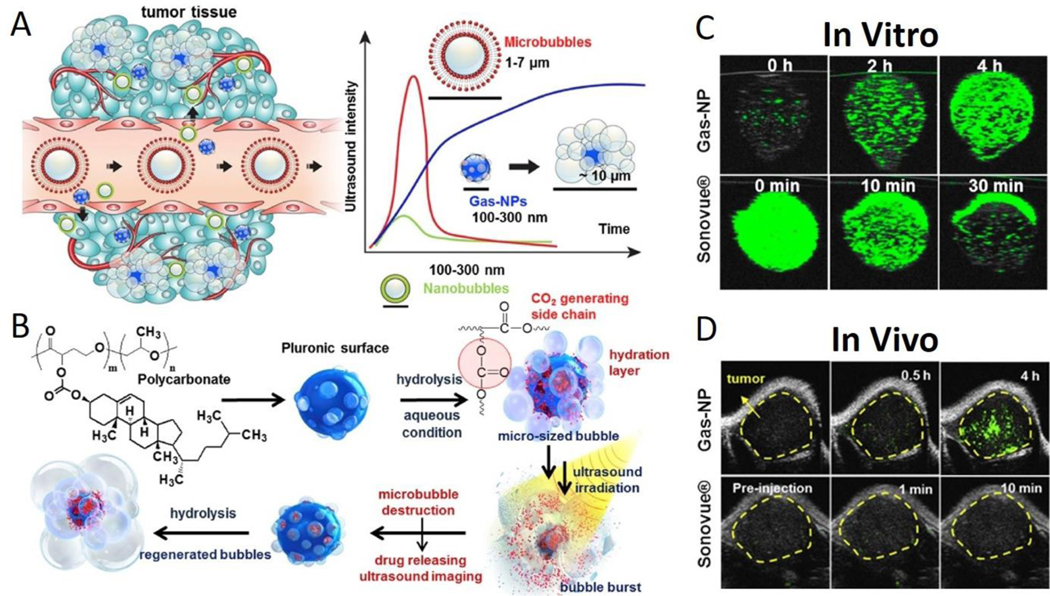
Fig. 7.
(A) Gas-NPs (blue nanoparticles) were synthesized via the O/W emulsion method with a size of 290nm and accumulated in tumor through the EPR effect. Current ultrasound contrast agents and large-sized perfluorocarbons-encapsulated microbubbles (red microparticles) present strong echo signals, but the large dimension prevents extravasation from vessel to surrounding tumor tissue. Also, small-sized nanobubbles (green nanoparticles) demonstrate a good biological distribution and effective extravasation, but the echo signals are not strong enough. (B) Chemical structure of hydrolysable carbonate copolymer; Poly(cholesteryl butyrolacone-co-propylene oxide). The carbonate copolymer was emulsified to produce solidified Gas-NPs via the O/W emulsion method. The Gas-NPs start to hydrolyze to produce CO2 nanobubbles in aqueous condition, followed by expansion/coalescence of nanobubbles into microbubbles for the targeted tumor US imaging. In addition, the anticancer drug-loaded Gas-NPs enable US-triggered drug delivery. (C) The US imaging test in vitro demonstrated a gradually CO2 generating process, current ultrasound contrast agent (Sonovue®) was the control (D) The ultrasound imaging ability in vivo showed a strong and persisted signals in whole tumor, current ultrasound contrast agent (Sonovue®) was the control. This nanoparticle demonstrates the unique and beneficial chemical gas-generating mechanism and is potentially useful for real-time ultrasound imaging and cancer therapy. Reproduced with permission.190 Copyright 2016. Elsevier.