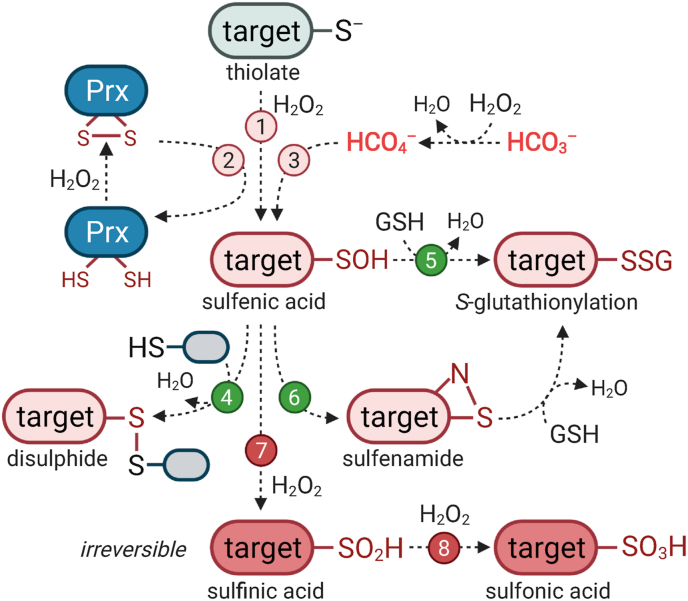
Fig. 1.
Potential redox modifications of cysteines by H2O2. Cysteine thiols (R–SH) are partially deprotonated at physiological pH to the thiolate state (R–S–). In the presence of H2O2, the thiolate can undergo initial oxidation to form sulfenic acid (R–SOH), through a range of mechanisms: (1) direct oxidation, (2) oxidation mediated by a highly redox-reactive second protein such as peroxiredoxin (Prx), or (3) after exposure to highly reactive compounds such as peroximonocarbonate (HCO4−), which is spontaneously generated in a reversible reaction between H2O2 and bicarbonate (HCO3−) (see also Fig. 2). Sulfenic acid is relatively reactive and can form inter- or intra-molecular disulphides with a second adjacent thiol (R–SS–R) (4). Alternatively, the sulfenic acid can react with a low molecular weight thiol, e.g. GSH results in S-glutathionylation (R-SSG) (5). In some proteins (e.g. PTP1B), an intermediate redox formation occurs, where the sulfenic acid reacts rapidly with a serine in close proximity to form a sulfenamide (R–SN) that can be further S-glutathionylated (6). These redox modifications are reversible and can be reduced back to the initial thiolate state by cellular antioxidant systems. However, under conditions of oxidative stress, the sulfenic acid can form higher oxidation states: (7) sulfinic acid (R–SO2H) and (8) sulfonic acid (R–SO3H), which are both irreversible modifications.