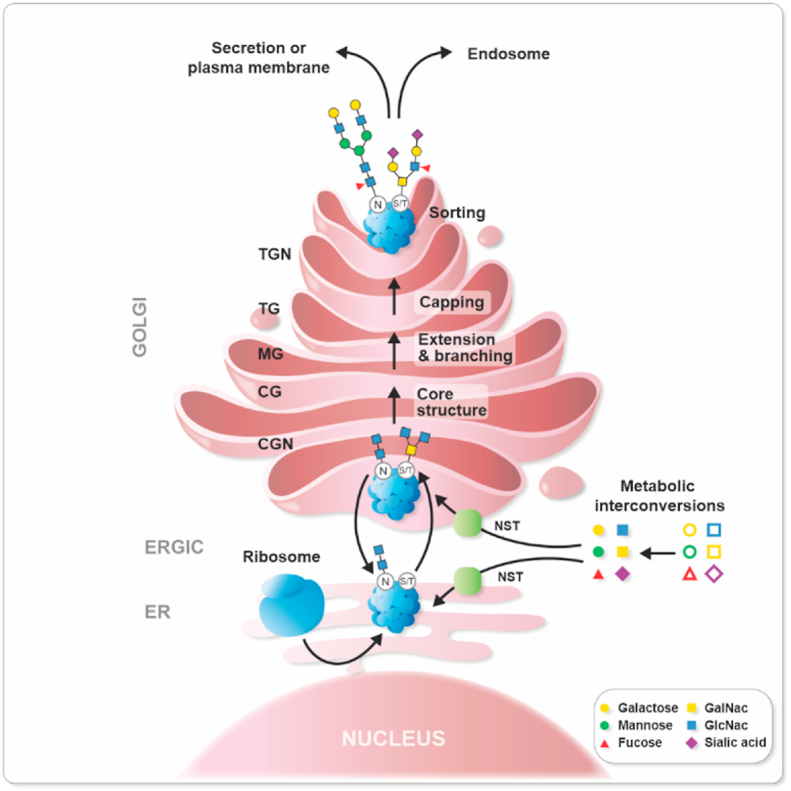
Fig. 1.
Glycoprotein formation. (A) First, cytosolic enzymatic reactions convert monosaccharides to “active sugars”, which are then translocated into the ER and Golgi via nucleotide sugar transporters (NST). The most frequently occurring glycans are N-glycans and O-glycans. In N-linked glycans the glycosylation process starts in the lumen of the ER where an oligosaccharyltransferase attaches a monosaccharide precursor to an amide nitrogen of an asparagine or arginine residue residing in a specific consensus sequence of the nascent protein. Further trimming of the glycan structures and control of misfolded glycoproteins in the ER is performed before the protein exits the ER-via the Golgi intermediate compartment (ERGIC). The O-linked glycosylation is a form of glycosylation that occurs in the ER or the Golgi where different sugars can be added to hydroxyl groups present in the amino acids serine, threonine, tyrosine, hydroxylysine, or hydroxyproline; alternatively oxygen present in lipids such as ceramide can be used. In the Golgi lumen, activated sugars are used as donors in sequential reactions with glycosyltransferases (GTs) which together with glycosidases (Gly) extend, and branch the glycan by involving the cis Golgi (CG), and medial Golgi (MG) before they are capped with terminal sugars such as sialic acid. Finally, the glycoprotein is sorted into a vesicle through the trans Golgi (TG) and the trans Golgi network (TGN) and transported to its final destination.