Abstract
Ventroposterior medialis parvocellularis (VPMP) nucleus of the primate thalamus receives direct input from the nucleus of the solitary tract, whereas the homologous thalamic structure in the rodent does not. To reveal whether the synaptic circuitries in these nuclei lend evidence for conservation of design principles in the taste thalamus across species or across sensory thalamus in general, we characterized the ultrastructural and molecular properties of the VPMP in a close relative of primates, the tree shrew (Tupaia belangeri), and compared these to known properties of the taste thalamus in rodent, and the visual thalamus in mammals. Electron microscopy analysis to categorize the synaptic inputs in the VPMP revealed that the largest-size terminals contained many vesicles and formed large synaptic zones with thick postsynaptic density on multiple, medium-caliber dendrite segments. Some formed triads within glomerular arrangements. Smaller-sized terminals contained dark mitochondria; most formed a single asymmetric or symmetric synapse on small-diameter dendrites. Immuno-EM experiments revealed that the large-size terminals contained VGLUT2, whereas the small-size terminal populations contained VGLUT1 or ChAT. These findings provide evidence that the morphological and molecular characteristics of synaptic circuitry in the tree shrew VPMP are similar to that in non-chemical sensory thalamic nuclei. Furthermore, the results indicate that all primary sensory nuclei of the thalamus in higher mammals share a structural template for processing thalamocortical sensory information. In contrast, substantial morphological and molecular differences in rodent versus tree shrew taste nuclei suggest a fundamental divergence in cellular processing mechanisms of taste input in these two species.
Keywords: ventral posteromedial parvocellular nucleus (VPMP), VGLUT1, VGLUT2, GAD65, GAD67, ChAT, electron microscopy, gustatory system, synaptic circuitry
Graphical Abstract
The synaptic circuitry of primary sensory thalamic nuclei has been well characterized and show striking similarities both in terminal morphology and chemical characteristics. The exception to this is the taste thalamus, the VPMP. A past study of synaptic circuitry in the rat taste thalamus (VPMpc) revealed marked differences from other nuclei like the LGN and MGN. Our data demonstrates that the tree shrew VPMP shares synaptic characteristics common to primary sensory thalamic nuclei including presynaptic interneuron dendrites, large VGluT2 positive terminals, and triadic arrangements in glomeruli.
1. INTRODUCTION
In the last half-century, studies using tract-tracing and immuno-electron microscopy have characterized the structural properties of synaptic connections within visual (Balaram et al., 2015; Bickford et al., 2000; Cavdar et al., 2011; Colonnier & Guillery, 1964; Erisir, Van Horn, Bickford, et al., 1997a; Erisir, Van Horn & Sherman 1997b; Erisir et al., 1998; Van Horn et al., 2000), auditory (Bartlett et al., 2000; Coomes et al., 2002; Jones & Rockel, 1971; Morest, 1975), and somatosensory (Jones & Powell, 1969; Liu et al., 1995; Ma et al., 1987; Ohara et al., 1989a) sensory thalamic nuclei in various species. These studies have revealed that the inputs to the sensory thalamus displayed remarkable similarities in synaptic characteristics across different senses and across mammalian species including primates, rodents, cats, and ferrets (reviewed in Sherman & Guillery, 2001). For example, in visual and auditory thalamic nuclei, the main excitatory input that has the capacity to drive thalamic cells to action potential (i.e., the driver retinal axons in the dorsal lateral geniculate nucleus (dLGN), and the driver axons from the central nucleus of the inferior colliculus to the ventral division of medial geniculate nucleus (vMGN) respectively), have a similar morphology and target similar postsynaptic elements (Bartlett et al., 2000; Guillery, 1969b; Jones & Rockel, 1971; McAllister & Wells, 1981). Characteristically, these driver axons make large-volume terminal boutons that contain pale mitochondria, form wide, asymmetric synaptic zones onto proximal portions of relay dendrites, and use VGLUT2 to exert glutamatergic function (Balaram et al., 2015; Guillery, 1969b; Kaas et al., 1972; Land et al., 2004; Rovo et al., 2012; Sherman & Guillery, 1998). Several other inputs, including inhibitory axons from GABAergic interneurons and the thalamic reticular nucleus (TRN), glutamatergic feedback (using VGLUT1) from layer VI of the cortex, and cholinergic input from the brainstem modulate or modify the excitability of sensory thalamic cells (Bartlett et al., 2000; Casagrande et al., 2006; Cavdar et al., 2011; Coomes et al., 2002; Erisir et al., 1997a; Govindaiah & Cox, 2006; Liu et al., 1995; McCormick, 1992; Ohara et al., 1989a; Wang et al., 2002). A group of these inputs selectively interact within glomerular arrangements and form triads where localized GABAergic influences can be exerted on relay cells (Famiglietti & Peters, 1972; Lam et al., 2005; Lieberman & Webster, 1974). Understanding the origin of input axons into the sensory thalamus, the molecular and morphological properties of synaptic terminals, and how those inputs interact with geniculate cells and with each other have provided insights into the function of thalamic sensory processing (Bickford, 2015, 2019; Guillery & Sherman, 2002; Sherman, 2007; Sherman & Guillery, 1996, 2002). The similarities in thalamic synaptic circuitry across vision, hearing, and touch in various mammals have been identified as indicators for a common and phylogenetically conserved mechanism to modify and modulate sensory information in the thalamus.
The fourth sense with an associated thalamic nucleus, taste, is the least studied and understood. In rodents, the species most commonly used to study taste behavior and neural coding, the function of the thalamocortical pathway is thought to be complementary to limbic, visceral and multisensory inputs that shape the neural function of insular cortex neurons (Allen et al., 1991; Cechetto & Saper, 1987; Maffei et al., 2012; Saddoris et al., 2009; Shi & Cassell, 1998). The synaptic circuitry in the rodent taste thalamus has been characterized using tract-tracing and immuno-electron microscopy (Holtz et al., 2015), revealing that the ventral posteromedial nucleus parvocellularis (VPMpc) receives its primary input via very large terminals that contain CGRP in dense-core vesicles that surround the most proximal portions of relay cell dendrites. Furthermore, the rodent VPMpc does not contain interneurons, glomeruli or triadic arrangements. In the study done by Holtz et al., the synaptic circuitry differences between rodent taste thalamus and other, non-chemical nuclei are attributed to potential differences in how taste information is coded through thalamocortical pathways. However, an alternative interpretation may deserve further exploration: Synaptic circuitry in the rodent VPMpc may be a downstream adaptation of other fundamental pathway differences that exist in rodents in comparison to primates (Beckstead et al.,1980; Benjamin & Burton, 1968; Pritchard et al., 1989; reviewed in Pritchard & Di Lorenzo, 2015).
In order to determine if synaptic properties seen in the rat VPMpc are an accurate representation of the taste thalamus in primates, we studied the cellular and morphological properties in VPMP, the taste thalamus of tree shrews, using electron microscopy (see Discussion for a review of the nomenclature used for referring to the taste thalamus in rodents and primates). Tree shrews are one of the closest living relatives to prosimian primates, diverged evolutionarily about 80 million years ago (Murphy et al., 2001), and about 20 million years after the rodents have (Nei & Glazko, 2002). As they are easily bred in captive colonies, and they share many common brain features with primates, the tree shrews have been used as animal models in a variety of biomedical and neuroscience research (Cao et al., 2003; Ranc et al., 2012; Rice et al., 2011; Wong & Kaas, 2009). In particular, the tree shrews have been a useful model in studies of sensory processing and the organization of the visual thalamus (Balaram et al., 2015; Baldwin et al., 2013; Day-Brown et al., 2017; Petry & Bickford, 2019; Van Hooser et al., 2013; Vanni et al., 2015; Fitzpatrick, 1996). The immunocytochemical, quantitative, and qualitative ultrastructural analyses reported here reveal that the tree shrew VPMP shares characteristics seen in other sensory thalamic nuclei, and that the tree shrew VPMP is likely to be a more representative model of primate taste processing in the thalamus and forebrain than that of rodents.
2. MATERIALS AND METHODS
Animals:
Data for this study were collected from the brains of six adult tree shrews (Tupaia belangeri) of both sexes. All procedures in this study were approved by the University of Virginia Institutional Animal Care and Use Committee (IACUC) and the University of Louisville IACUC, and conform to the National Institutes of Health guidelines.
Tissue Preparation:
Animals were deeply anesthetized with an overdose of euthasol (excess of 0.25mL/kg i.p.) and transcardially perfused with Tyrode’s solution (1-2 min) followed by 300mL of a fixative solution containing 4% paraformaldehyde, and 0.5% or 2% glutaraldehyde in 0.1M phosphate buffer (pH 7.4). Brains were removed and post-fixed overnight in the fixative solution at 4°C. Subsequently, brains were blocked and sectioned at 60μm using a vibratome, and collected in six series. Two of the series (#1 and #4) were mounted on subbed slides for histochemical stains. One of the series was resin embedding for EM analysis. All other sections were rinsed in 1% sodium borohydride and stored in 0.05% azide in 0.01M PBS at 4°C, prior to immunocytochemistry.
Histochemistry:
For visualization of cytochrome oxidase, free-floating sections were incubated in a solution of 10mg sucrose and 0.5ml DMSO in 100ml PB for ten minutes at room temperature. This was followed by overnight incubation in 30 mg cytochrome C type III and 50 mg DAB in 100mL 0.1M PB in a dark 40°C oven. For myelin visualization, sections that were mounted on subbed slides were rehydrated in 0.02M PBS for 2 min and incubated in 0.2% HAuCl4 for 10-15 min at 60°C (Corson et al., 2012). Once fine myelinated fibers were differentiated, the slides were transferred in an intensification solution of 0.2% KAuCl4 for 2-3 min at 60°C, followed by two rinses in 0.02M PBS for 2 min each. Finally, sections were incubated in 1% sodium thiosulfate for 3 min and rinsed three times in 0.02M PBS. Then the slides were treated through a series of ETOH for dehydration, and through xylenes for clearing the tissue of lipids. All slides were coverslipped using the DPX mounting media (Sigma Aldrich, St. Louis, MO).
Embedding for Electron Microscopy:
Using routine protocols, sections were treated with 1% Osmium tetroxide in 0.1M PB for one hour. They were then en bloc counterstained with filtered 4% uranyl acetate in 70% alcohol for one hour, dehydrated with in series of alcohol and acetone, and infiltrated with resin (EMbed 812, EMS, Hatfielsd, PA) overnight. Sections were then flat embedded between two Aclar sheets (EMS, Hatfield, PA), and were cured in a 60°C oven 1-2 days. Sections containing VPMP were identified from flat-embed sections and photographed using a light microscope. The area of interest from each section was excised and placed in BEEM capsules (EMS, Hatfield, PA). The capsules were filled with resin and cured at 60°C for another 1-2 days, or until polymerized. The area of interest on the capsule was traced with a camera lucida and trimmed to a 1mm by 2mm trapezoid, usually containing the entirety of VPMP. Ultrathin sections of 50-80 nm thickness were collected on 200 mesh copper grids (Ted Pella, Redding, CA) using an ultramicrotome (Ultracut UCT7; Leica, Buffalo Grove, IL).
Immunohistochemistry:
Table 1 lists the primary and secondary antibodies and the dilutions used in the current study. Terminals positive for vesicular glutamate transporter type 2 were identified with guinea pig polyclonal anti-VGLUT2 (EMD Millipore, Burlington, MA). This antibody identifies the C-terminal sequence of rat VGLUT2, as verified in Zelano et al. (2009). Terminals containing vesicular glutamate transporter type-2 were identified with a guinea pig polyclonal antibody for rat VGLUT1 (EDM Millipore, Burlington, MA). This antibody does not have any cross-reactivity with VGLUT2, as verified in Persson et al. (Persson et al., 2006). Cholinergic terminals were identified with rabbit polyclonal anti-ChAT (EDM Millipore, Burlington, MA), which identifies a peptide sequence from porcine ChAT (Myöhänen et al., 2008). GABAergic interneurons were identified with rabbit polyclonal anti-GAD67 (Novus Biologicals, Littleton, CO) made from synthetic human GAD67. This antibody is verified in a study by Agca et al. (2014). Finally, GABAergic terminals were identified with rabbit polyclonal anti-GAD65 (Sigma Aldrich, Burlington, MA) verified in a study by Tabor et al. (2011).
TABLE 1.
Antibodies Used in this Study
| Antibody Name | Immunogen | Antibody Info | Dilution |
|---|---|---|---|
| Anti-Vesicular glutamate transporter 2 (VGLUT2) | KLH-conjugated linear peptide corresponding to the C-terminal sequence of rat VGLUT2. | EMD Millipore Corporation; Cat# AB2251; RRID:AB_1587626; Guinea Pig (Polyclonal) | 1:2,500 |
| Anti-Vesicular glutamate transporter 1 (VGLUT1) | Synthetic peptide from rat VGLUT1 protein with no overlap to VGLUT2. | EMD Millipore Corporation; Cat# AB5905; RRID:AB_2301751; Guinea Pig (Polyclonal) | 1:5,000 |
| Anti-Choline Acetyltransferase (ChAT) | A 22 amino acid synthetic peptide from porcine ChAT, coupled to KLH. The peptide sequence is GLFSSYRLPGHTQDTLVAQKSS. | EMD Millipore Corporation; Cat# AB5042; RRID: AB_91650; Rabbit (Polyclonal) | 1:2,000 |
| Anti-Glutamic acid decarboxylase 67 (GAD67) | KLH-conjugated synthetic peptide corresponding to a portion of amino acids 1-100 of human GAD67. | Novus Biologicals; Cat# NB100-56385; RRID: AB_838284; Rabbit (polyclonal) | 1:2,000 |
| Anti-Glutamic acid decarboxylase 65 (GAD65) | KLH-conjugated linear peptide corresponding to human GAD65 | Millipore Cat# AB5082, RRID:AB_2107925; Rabbit (polyclonal) | 1:1,000 |
| Secondary Antibodies | |||
| Biotin anti-Guinea Pig IgG | n/a | Vector; Cat# BA-7000; Goat (polyclonal) | 1:50 |
| Biotin anti-Rabbit IgG | n/a | Vector; Cat# BA-1000; Goat (polyclonal) | 1:50 |
| Alexa Flour 488 anti-Rabbit IgG | n/a | ThermoFisher Scientific; CAT# A32731; Goat (polyclonal) | 1:50 |
For pre-embedding immunostaining for electron microscopy, the sections were pre-incubated in 1% BSA in 0.01MPB with 0.06% triton and 0.05% sodium azide for 30 min, and they were then transferred to primary antibodies anti-VGLUT2, anti-CGRP, or anti-VGluT1 in 1% BSA-PBS and 0.05% sodium azide for 72 hr on a shaker. Sections were rinsed in 0.01M PBS to terminate the incubation and were transferred to secondary antibody conjugated to biotin for 2 hours, followed by a treatment avidinbiotin-complex (ABC; Vector) solution for 2 hours. The tissue was then rinsed in 0.01M PBS and incubated in a solution of hydrogen peroxide and 0.05% diaminobenzidine for 3-7 minutes.
For confocal microscopy immunostaining, the sections were incubated in the primary antibodies (anti-GAD65 or anti-GAD67) that were diluted in 0.01M PBS containing 1% BSA, 0.3% triton and 0.05% sodium azide for 48 hours on a shaker. Sections were then rinsed and incubated in the secondary antibody conjugated to Alexa Flour 488 for 2 hours. Sections were mounted onto glass slides and cover-slipped using anti-fade mounting medium.
Imaging and Analysis:
To obtain images for light microscopy figures, the sections were photographed using a Leica microscope (model LMDC 888011) and Leica MC170 digital camera. Confocal images were captured using a Nikon 80i microscope fitted with a Nikon C2 Scanning System (Nikon Instruments) and a 20× objective and a 488 nm, 10mW argon laser. Photographs were then annotated using Adobe Photoshop software.
For electron microscopy analysis, the ultrathin sections on copper grids were examined on a JEOL1010 electron micrograph equipped with a 16-megapixel CCD camera (SIA). Images for quantitative morphology and immuno-labeled terminal analysis were taken at 10,000X magnification, yielding 2.75pixels/nm resolution that is sufficient for clear visualization of lipid bilayer. For morphology analysis using unstained sections, near-overlapping EM images were captured at 10,000X magnification along strips that spanned the long (mediolateral) and short (dorsoventral) axes of VPMP. These images were then stitched to create composite strips or areas across the nucleus. Image Pro Plus 7 (Media Cybernetics, Silver Spring, MD) was used to quantify terminal area, dendrite caliber, and synapse length. These attributes were outlined, and synapses were traced. Postsynaptic dendrite caliber was measured using the “feret minimum” (feretmin) measurement module of Image Pro Plus. This measurement allows for the measurement of irregularly shaped dendritic cross-sections using an algorithm that places a rectangle around the dendrite; the long and short sides of the rectangle yield measures of how obliquely the dendrite was cut and the dendrite caliber, respectively.
The statistical analysis for non-parametric testing, including Mann-Whitney U tests and descriptive statistics was done using GraphPad Prism software (GraphPad.com). Multimodal distribution analysis for terminal size was conducted using the MClust package in R (version 3.6.2). All figures and graphs were created using Prism, RStudio, Adobe Illustrator, and Adobe Photoshop.
The data that support the findings of this study including raw electron microscopy images and quantitative data are available from the corresponding author upon reasonable request (Data Availability Statement).
3. RESULTS
Architectonic and histochemical borders of the tree shrew VPMP
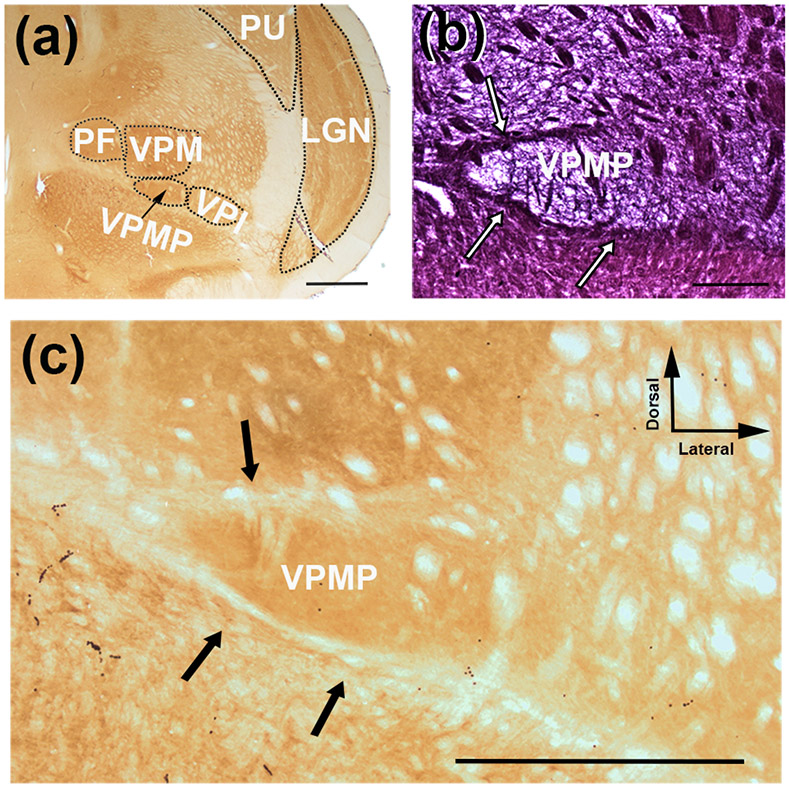
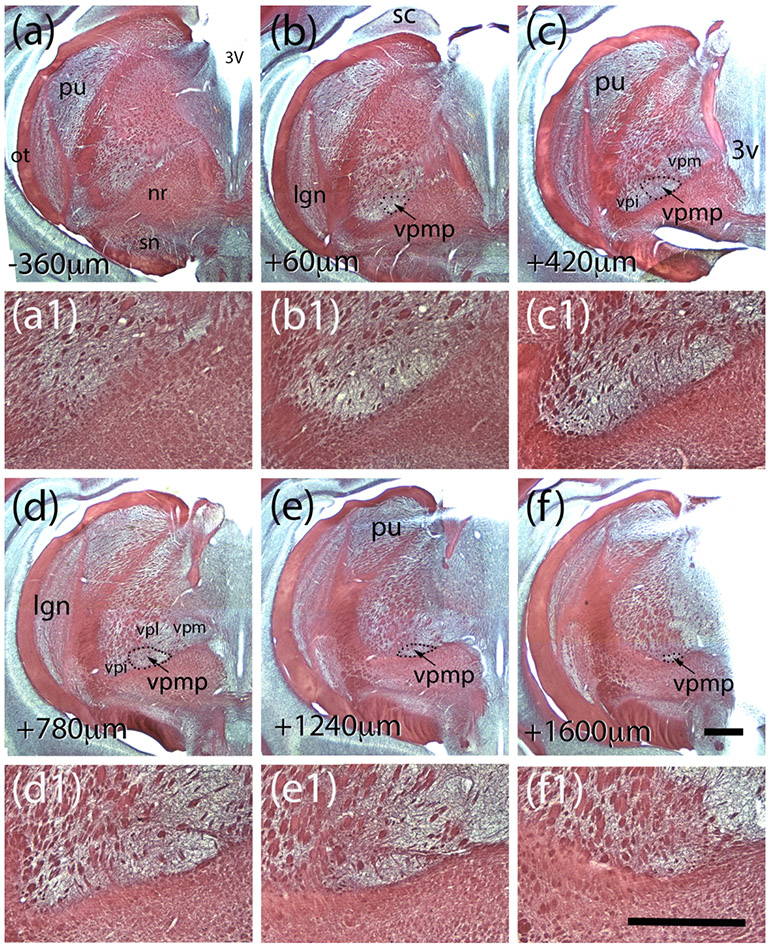
In order to describe cytoarchitectural boundaries of the taste thalamus of the tree shrew, we examined serial coronal sections of the thalamus using histochemical techniques including myelin and cytochrome oxidase (Figure 1 a-c). Both the myelin and the cytochrome oxidase stains prominently define the medial, dorsal and ventral aspects of a triangular-shaped nucleus that is surrounded by VPM dorsally, ventral posterolateral nucleus (VPL) laterally, perifascicular nucleus (PF) medially, and the ventral medullary laminae of the thalamus (LMV) ventrally (Figure 1 a). This region was identified as the VPMP using an atlas of the tree shrew brain (Tigges & Shantha, 1969), and it is located about 2 mm lateral from the midline and 7.5 mm ventral from the brain surface (Figure 2 a-f). The posterior-most sections of VPMP are encountered in the anterior-most aspect of the superior colliculus in coronal sections (Figure 2 b-b1). Its triangular shape is most prominent in coronal sections, in which the 3rd ventricle courses along the midline (Figure 2 c-e). The VPMP becomes undifferentiated as the dLGN in the same coronal section becomes confined to its dorsal aspects (Figure 2 f-f1). The VPMP extends about 1.5 −1.8 mm from the most rostral to the most caudal point (Figure 2). The medial tip of the triangular nucleus is most prominently delineated by a myelinated fiber bundle (Figure 2c1-f1). A few patches of myelinated axon bundles traverse the nucleus dorsoventrally, and fine myelinated fibers crisscross through the nucleus (Figure 1 b; Figure 2b1-e1).
Figure 1.
Coronal sections of the VPMP in the tree shrew stained for cytochrome oxidase (a,c) and myelin (b). VPMP, similar to other sensory nuclei, displays high cytochrome oxidase activity (a,c). Myelin histochemistry reveals myelinated axon bundles that demarcate VPMP at the dorsal, medial and ventral aspects (b; white arrows), which are lightly stained with cytochrome oxidase (c, black arrows). The tree shrew VPMP is ventral to the VPM, lateral to the PF and medial to the VPI (a). Scale bar = 1000 μm in a and c; and 600 μm in b.
Figure 2.
Myelin-stained coronal sections of tree shrew brain reveal the landmarks and relative coordinates for the tree shrew VPMP. The sections in panels a - f are 360 μm apart, and they are organized from posterior to anterior. The distances from the first section that displayed VPMP borders are indicated at lower left corners. The panels a1-f1 are higher magnification views of the VPMP from the corresponding panels above. Abbrv: 3V: third ventricle; lgn: dorsal lateral geniculate nucleus; nr: red nucleus; ot: optic tract; pu:pulvinar; sc: superior colliculus; sn: substantia nigra; vpi:n. ventral posterior inferior n.; vpmp: n. ventralis posterior medialis pars parvocellularis; vpm: n. ventralis postrior medialis; vpl: n. ventralis posterior lateralis. Scale bar in panel f = 1mm and applies to a-f. Scale bar in panel f1 = 1mm and applies to a1-f1.
Morphological properties of tree shrew VPMP ultrastructure
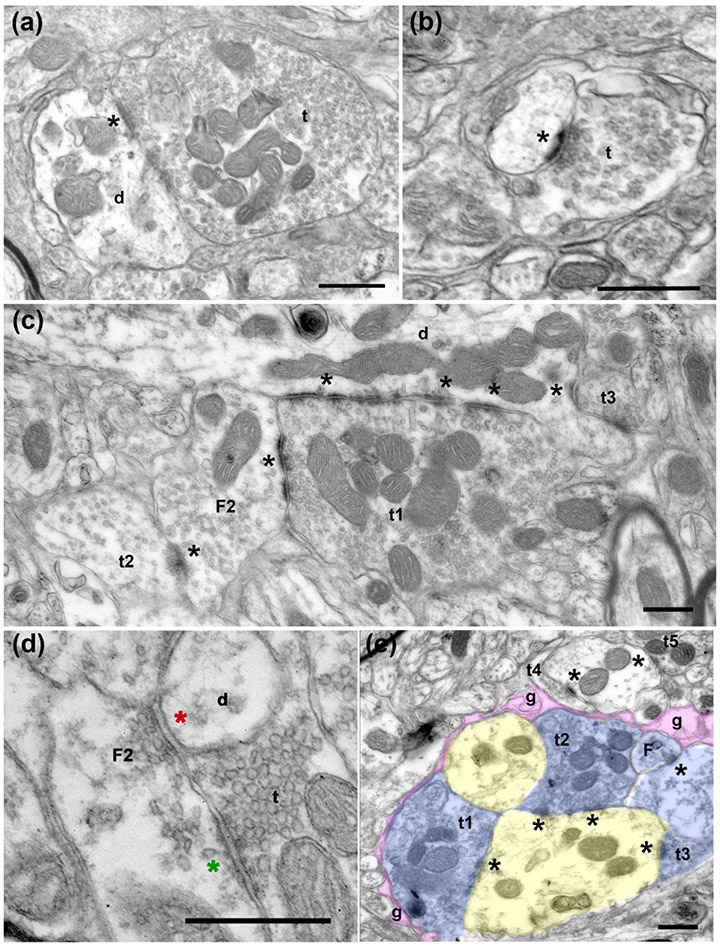
To classify the inputs into the VPMP based on their morphological properties, we examined a total of about 1700 μm2 area from the VPMP of three tree shrew brains at a resolution that allows for the differentiation of synaptic structures. Dendrite segments in the VPMP receive synapses from terminals of various sizes (Figure 3 a-c). Postsynaptic dendrites often contain mitochondria and prominent postsynaptic density at the sites of synapses (Figure 3 a-c).
Figure 3.
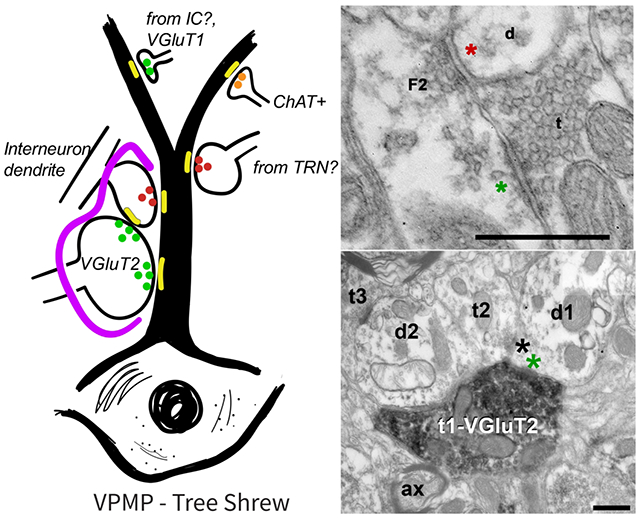
Electron micrographs of terminals in the tree shrew VPMP. (a) A large terminal (t) filled with round vesicles and many mitochondria makes a synapse onto a large dendrite (d). The asterisk (*) marks the synapse at the postsynaptic side, here and in all subsequent panels. (b) A small terminal (t) with sparse vesicles synapses onto a dendrite. (c) A large terminal (t1) filled with round vesicles and many mitochondria makes a synapse onto a dendrite (d) and a vesicle filled profile (F2; classified as such because it contains pleomorphic-vesicles and it is postsynaptic to another terminal). Note that both synapses display multiple active zones (*). Another terminal (t2) with sparse pleomorphic vesicles (presumed inhibitory) synapses onto the same F2 profile, representing an inhibitory-to- inhibitory interaction. A third terminal (t3) that is notably small compared to t1, synapses onto the large dendrite. (d) A terminal (t) with round vesicles synapses onto a presynaptic dendrite (F2). The green asterisk marks the putative excitatory synapse. The presynaptic dendrite (F2), in turn, forms a symmetrical, presumed inhibitory synapse (red asterisk) onto another dendrite (d). (e) In a glomerular structure, three terminals (t1, t2, t3) that are pseudo-colored in blue form synapses onto a large dendrite (yellow). The synaptic arrangement is encased in an astrocytic sheath (g, pink). The synapses are indicated by asterisks at the postsynaptic side. Scale bars= 500 nm.
Terminals in the VPMP display a range of morphological characteristics. The most common terminal bouton type has small cross-section area, contains densely packed vesicles, dark mitochondria (although rare) and forms a single synapse (Figure 3 b,c, e). The synapses formed by these small terminals often display thick postsynaptic density (Figure 3 b). Larger cross-section areas characterize another distinct type of terminal encountered in the tree shrew VPMP, and these contain round vesicles and many mitochondria (Figure 3 a). The synapses of these large terminals can make multiple synaptic zones and display asymmetric morphology (Figures 3 a, c). Large terminals can form multiple synapses on more than one dendrite segment. The profiles that are postsynaptic to these terminals are either dendrites of various calibers or small protrusions that emerge from dendrites (Figures 3 a, c).
The tree shrew taste thalamus contains glia-encapsulated glomerulus structures containing synaptic triads, a distinct morphological entity described as a major characteristic in the sensory thalamus (Somogyi et al., 1978; Hajdu et al., 1982; Liu et al., 1995; Bickford 2019). Triadic arrangements in the tree shrew VPMP consist of a large bouton synapsing onto a dendrite and onto another medium-sized profile that contains vesicles and, in turn, forms a synapse onto the same dendrite as the large bouton (Figure 3 e). This terminal type, a vesicle-containing profile that can be both presynaptic and postsynaptic, has been described in the dLGN, MGN, and VPM of all species examined so far, and has been termed “F2 type” terminals (Bickford et al., 2010; Coomes et al., 2002; Montero, 1986; Ohara et al., 1989b; Sherman & Guillery, 2001; Wang et al., 2002). F2 type terminals have been confirmed to be dendritic appendages of GABAergic interneurons (Montero, 1986).
Quantitative properties of the tree shrew VPMP ultrastructure
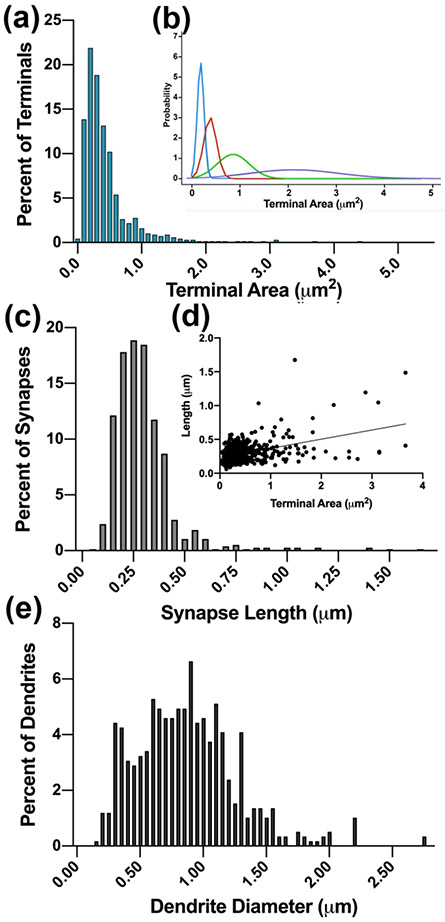
The measurements of terminal bouton cross-section area can be used to differentiate distinct inputs to the thalamus (Erisir et al., 1997b; Guillery, 1969a; Van Horn et al., 2000). To determine the quantitative distribution of terminal bouton sizes and to reveal whether the tree shrew VPMP has inputs with distinct terminal sizes, we analyzed the size distribution of terminal cross-section areas. For this analysis, at least 50 minimally overlapping images from each of three tree shrew VPMP were examined using Image Pro Plus, and every terminal that displayed a synapse at the cross-section were marked and measured, yielding a final data set that included 685 terminals. The cross-section areas of VPMP terminals ranged from 0.030 μm2 to 4.373 +/− 0.452 μm2 (mean= 0.449 μm2; median=0.323 μm2), displaying a multimodal distribution formed by at least four distinct populations (Shapiro-Wilk normality test, P<0.0001) (Figure 4 a-b). The great majority of the terminals were small: 74.3% of all terminals were smaller than 0.5 μm2, and 91.7% of terminals were smaller than 1 μm2. The terminal cross-section size distribution in the tree shrew VPMP mirrors that in the visual thalamus: an axon population bearing very large terminal boutons brings the sparsest input while small- and medium-sized terminals form the overwhelming majority of synapses.
Figure 4.
(a). The frequency distribution histogram of terminal cross-section areas reveals multimodal population. (b). Four subpopulations revealed by a BIC analysis (R-MClust) are fitted as curves and plotted in different colors. (c). The frequency distribution histogram of synapse lengths. (d). A pairwise plot of terminal cross-section areas and the length of the synapses formed by each terminal (•), and the best fit linear correlation line. (e) The frequency distribution histogram of the calibers of dendrites that are postsynaptic to terminals in the dataset.
To determine if a correlation exists between the sizes of terminals and the size of the synapses they form, we measured the length of synapses in our data set (758 synapses from 3 tree shrew brains). The average length of synapses was 0.298 μm +/− 0.161 μm, ranging between 0.061 μm and 1.674 μm (Figure 4 c). The terminal area and synapse length are moderately and positively correlated (Pearson r= 0.44, n=727 pairs; Figure 4 d); as the terminal bouton size and the capacity for the vesicles grew, so did the neurotransmitter release zones. Only 3% of the synapses were larger than two standard deviations above the mean.
In visual, auditory, and somatosensory thalamic nuclei, axons originating from different neuron groups may selectively synapse on distinct neuron types or distinct segments of their dendritic tree, shaping the unified responsiveness of the postsynaptic cells (Bartlett et al., 2000; Holtz et al., 2015; Van Horn et al., 2000). In order to ascertain whether different inputs have selectivity for different dendrite segments in the tree shrew VPMP, we measured the caliber of the postsynaptic dendrites. We assumed that thicker caliber dendrites are proximal to the cell body and that smaller caliber dendrites are secondary or tertiary branches that are distal to the cell body. In a dataset of 587 postsynaptic dendrites in the tree shrew VPMP, the average dendrite diameter was 0.87μm +/− 0.4, ranging between 0.16 μm and 2.77 μm (Figure 4 e). Unlike as previously described in the rodent VPMpc (Holtz et al., 2015), we did not observe any terminals that synapsed on the dendrite segments emerging from the soma as evidenced by the lack of somatic organelles in the largest observed postsynaptic dendrites. While there was no correlation between the terminal size and the dendrite caliber (Pearson r=0.15, n=564 pairs) in general, the dendrites contacted by terminals that have the smallest 20% terminal size were statistically different than those contacted by the largest 20% of the terminals (Mann Whitney-U, p<0.0001). This suggests that a selectivity may exist for the smallest and the largest terminals for synapsing on the proximal or the distal portions of the dendrites.
Neurotransmitter properties of tree shrew VPMP:
To identify whether axonal inputs into the VPMP use distinct glutamate transporters, VGLUT1 and VGLUT2 immuno-electron microscopy was used. VGLUT1 is reactive in terminals from the cortex into the thalamus, and VGLUT2 is indicative of the main excitatory input into the thalamus from sensory drivers (Herzog et al., 2001; Rovó et al., 2012). With light microscopy, VGLUT2 immunohistochemistry reveals darkly labeled axonal swellings and large puncta in the VPMP (Figures 5 a-b). At the electron microscopy level, large VGLUT2 positive terminals are densely filled with dark chromogen of DAB, round vesicles, and many large mitochondria (Figures 5 c-d). Labeled terminals are also found in glomerular structures encapsulated in glia.
Figure 5.
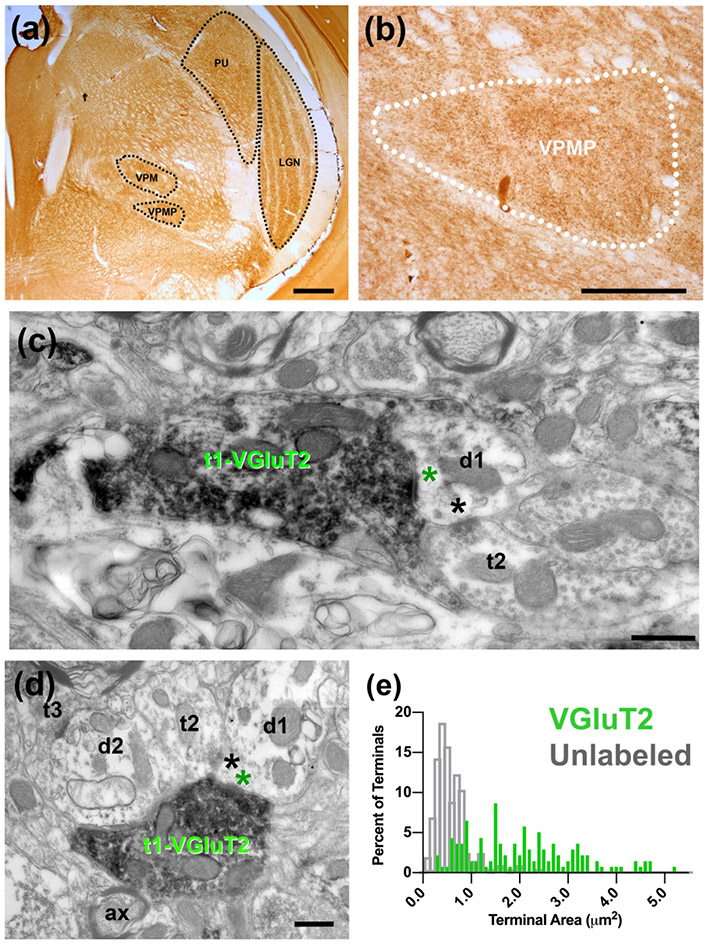
(a) Immunolabeling with VGLUT2 antibody delineates nuclei of the tree shrew thalamus (black outlines), including the dLGN, PU, VPM, and VPMP. (b) VGluT2 labeling of tree shrew VPMP (white outline) reveals dark puncta labeling throughout the VPMP. (c) Electron micrograph of immunolabeling with VGLUT2 antibody in terminals in the tree shrew VPMP. A large terminal (t1-VGluT2) with several many mitochondria displays VGLUT2 label primarily around vesicles; it makes an excitatory synapse (green asterisk) onto a dendrite (d1). An unlabeled terminal (t2) is synapsing (black asterisk) onto the same dendrite segment. (d) Triadic arrangement of terminals in the tree shrew VPMP. A VGluT2 positive terminal (t1-VGluT2) synapses (green asterisk) onto a presumed inhibitory terminal (t2) and a presumed relay cell dendrite (d1). The inhibitory terminal then synapses (black asterisk) on the relay cell dendrite. (e) The distribution histograms of terminal bouton area of VGluT2 labeled terminals (green) and unlabeled terminals (grey) in the tree shrew VPMP. Scale bar = 500 μm in panels (a) and (b); 500nm in panels (c ) and (d).
In order to reveal whether or not the VGluT2 terminals comprise the largest-sized inputs to VPMP, we examined the ultrathin sections at the EM and imaged every VGluT2 positive terminal encountered within VPMP borders. In a sample of 139 VGLUT2 positive terminals, the average cross-section area of terminals was 2.06 μm2 +/−1.12 μm2, ranging between 0.302 μm2 and 5.212 μm2 (Figure 5 e). Within this sample of VGluT2 positive terminals, 96.4% of all terminals were larger than 0.5 μm2, 77.7% were larger than 1μm2. Thus, in comparison to size distribution of all terminals in the VPMP (Figure 4 a), VGluT2+ terminals constitute the largest-sized population there. Because only ~8% of all VPMP terminals were larger than 1 μm2, we estimate that VGluT2+ terminals constitute a sparse input.
We also measured the terminal cross-section area of terminals that appeared in the same images but were not positive for VGluT2 (VGluT2-unlabeled terminals; Figure 5 e gray bars). The VGluT2-unlabeled terminal sizes range between 0.08μm2 and 2.43μm2 (mean=0.49μm2), and only 8.37% of these terminals are larger than 1 μm2. VGluT2 positive terminals are statistically larger than the VGluT2-unlabeled terminal populations (Mann-Whitney U test, P<0.001).
The synaptic zone sizes of VGLUT2 -positive terminals are also distinct when compared to the VGluT2-unlabeled terminals. We measured the length of 247 synapses formed by VGLUT2 positive terminals in the tree shrew VPMP. The synapse length of VGluT2-positive terminal ranges between 0.102 μm and 2.936 μm (mean= 0.570 μm ± 0.377 μm), and these are statistically larger than that of unlabeled terminals, which range in size from 0.11μm to 1.82 μm (Mann-Whitney U test, P<0.001).
The same pattern holds for dendrite selectivity of VGLUT2 labeled vs. unlabeled terminals. The caliber of dendrites targeted by VGluT2 labeled terminals (n=143) ranges between 0.193 μm and 3.797 μm (mean= 1.362 μm +/− 0.621 μm), and this is statistically larger than the caliber of dendrites targeted by the unlabeled terminals (Mann-Whitney U test, P<0.001; mean= 1.02 μm +/− 0.4 μm2, n=183).
Light microscopic analysis of VGLUT1 staining showed punctate labeling in the tree shrew VPMP, as well as in the dLGN. At the electron microscopy level, terminals appeared small with round vesicles that often synapsed onto thin postsynaptic dendrites (Figure 6a). Two or more of these terminals were often observed to synapse onto the same post-synaptic dendrite (Figure 6a). The average area of VGLUT1 labeled terminals was 0.299 +/− 0.133 μm2, with a minimum of 0.07 μm2 and a maximum of 0.61 μm2 (Figure 6c). The dendrites synapsed by VGluT1 positive terminals were thin, ranging between 0.3 and 1.2 μm in diameter (mean=0.87 +/− 0.2 μm2; n=48).
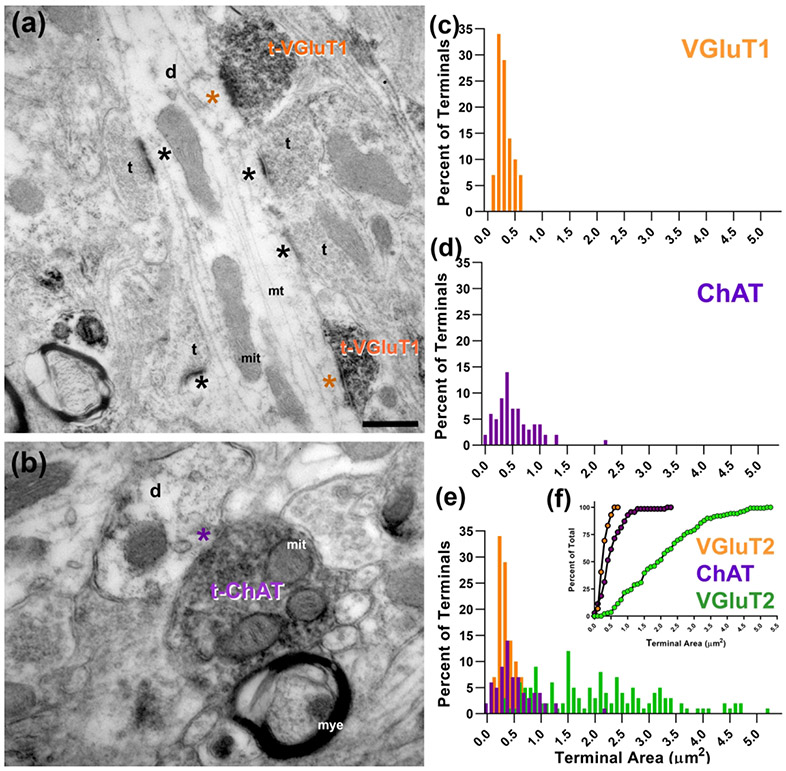
Figure 6.
(a) VGLUT1 immunochemistry revealed labeled terminals (t-VGluT1) in the tree shrew VPMP that were often seen synapsing (orange asterisk) on medium or small caliber dendrites (d) that also receive other labeled inputs and other small terminals (t; black asterisk). (b) Cholinergic terminals (t-ChAT) also make synapses (purple asterisk) onto small or medium sized dendrites (d). (c) The size distribution histogram of VGluT1 labeled terminals in the tree shrew VPMP. (d) The size distribution histogram of ChAT labeled terminals in the tree shrew VPMP. (e, f) Terminal size distribution (E) and cumulative probability (F) of VGluT2 (green), VGluT1 (orange), and ChAT (purple) labeled terminals in the tree shrew VPMP compare the distinct size characteristics of each terminal type. Scale bars = 200 nm; mit: mitochondria; mye: myelin sheath.
Cholinergic fibers that originate from parabrachial nucleus terminate on relay cell dendrites of visual and auditory relay cells (Erisir et al., 1997a,b; McCormick & Prince, 1987). To identify cholinergic terminals in the tree shrew VPMP, we labeled tissue with an antibody for choline acetyltransferase (ChAT), an enzyme that synthesizes acetylcholine in the terminal. At EM, the terminals that are positive for ChAT appear small, bear round vesicles and have few mitochondria (Figure 6 b). The terminal area of ChAT positive terminals range between 0.03 μm2 and 2.19 μm2 (mean= 0.54 μm2 +/− 0.36 μm2; n=70, Figure 6 d). The calibers of dendrites postsynaptic to cholinergic terminals range between 0.19 μm and 2.19μm (mean= 0.64μm +/− 0.43; n: 67); this is significantly larger than the size of dendrites contacted by VGluT1-positive terminals. The ChAT labeled terminals are significantly smaller than VGLUT2 labeled terminals (Mann-Whitney U test, P<0.001), but significantly larger than VGLUT1 labeled terminals (Mann-Whitney U test, P<0.001) and thus, all three make up distinct populations in the VPMP (Figure 6 e-f).
Calcitonin gene-related peptide (CGRP), which selectively marks the rodent VPMpc (Holtz et al., 2015), was not detected in the tree shrew VPMP in our immunocytochemistry experiments.
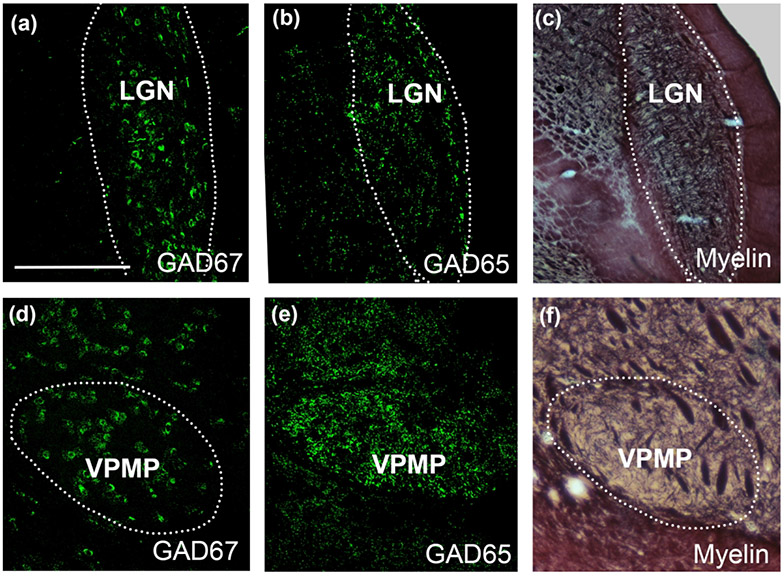
The presence of F2 terminals among VPMP ultrastructure implied the presence of GABAergic interneurons in tree shrew taste thalamus. In contrast, the rodent taste thalamus, as well as the most of ventral thalamic nuclei, is devoid of GABAergic cells (Barbaresi et al., 1986). In order to confirm the presence of inhibitory interneurons in the tree shrew VPMP, we used a GAD67 antibody to identify GABAergic cell bodies and GAD65 antibody to identify GABAergic terminals at the light microscopy level. This revealed that the tree shrew VPMP contained both axon terminals and cell bodies that are immunoreactive for anti-GAD65 and GAD67 labeling respectively (Figure 7). The surrounding ventral thalamic nuclei also contained GABAergic cells, adding to the similarities of the tree shrew and primate thalamus circuitries (Agarwala et al., 1992; Arcelli et al., 1997; Holdefer et al., 1988; Ohara et al., 1989a; Y. Smith et al., 1987).
Figure 7:
Immunolabeling with GAD67 reveals cell bodies in the dLGN (a) and the VPMP (d). Similarly, GAD65 immunolabeling reveals large puncta throughout in the dLGN (b) and VPMP (e) of the tree shrew thalamus. The myelin-stained sections (c,f) are useful to confirm the borders of thalamic nuclei imaged at the confocal microscope. The sections that are used to outline the dLGN and VPMP in panels (c) and (f) are adjacent to sections in panels (b) and (d), respectively. Scale Bar in a = 1 mm and applies to all panels.
4. DISCUSSION
The current study characterized the histological and morphological properties of the inputs to the tree shrew taste thalamus and compared them with the known features of the rodent taste thalamus, and with visual, auditory, and somatosensory nuclei in the mammalian thalamus. The study provided evidence that the inputs to the VPMP had characteristics that matched visual, auditory and somatosensory thalamic nuclei in a variety of mammalian species and that these common features were distinct from the taste thalamus in rodents. These results have two major implications: First, the interspecies difference between rodents and tree shrews suggests that the tree shrew can be a model of choice in studying the unique mechanisms of thalamocortical taste processing in a system analogous to humans. Second, the finding that the taste thalamic nucleus harbors morphological motifs that are hallmarks of inputs to its visual, auditory, and somatosensory counterparts suggests more commonalities between these senses than previously appreciated and may contribute to the quests to understand the role of the thalamus in cortical function.
Taste thalamus nomenclature in rodents and primates.
The thalamic nucleus that receives and relays taste information has been given several names since it was recognized as a distinct structure, including VPMpc, VPMP, and VPMp in rodents, cats and primates (Beckstead et al., 1980; Hannig & Jürgens, 2006; Iyengar et al., 2007; E. G. Jones, 1985; Lin et al., 1979; Manocha et al., 1967; Yasui et al., 1983). In order to disambiguate the nomenclature used for the taste relay from the other ventromedial complex nuclei that are activated by a somatosensory stimulus (Blomquist et al., 1962; R Emmers, 1966; Raimond Emmers et al., 1962), E.G. Jones coined a new term in the first edition of The Thalamus (E. G. Jones, 1985): basal ventral medial nucleus (VMb) for referring to the taste relay. Adopted by a few authors since then (Clascá et al., 1997; Craig, 2002), VMb remains another viable alternative for referring to the taste relay of thalamus, particularly in human literature. In our study, we adopted the nomenclature and the abbreviation, VPMP that was used in the 1969 atlas of the tree shrew brain (Tigges & Shantha, 1969).
Ultrastructure morphology of the tree shrew VPMP
The tree shrew VPMP receives several types of input axons that are distinct both in their terminal bouton morphology and in their synaptic arrangements. These include: 1) Large terminals, dense with vesicles and utilizing VGLUT2. These terminals are sparse and make synapses on larger caliber dendrites. 2) Small terminals with dark mitochondria and round vesicles. These either use VGluT1 or ChAT and have a preference for smaller caliber dendrites. 3) Medium-size terminals that can also be postsynaptic to other large- or medium-size terminals. Similar to those termed “F2” in other sensory thalamic nuclei, these terminals engage in triadic arrangements in glia-encapsulated glomerular zones.
Thus, a main conclusion to be drawn here is that there is an almost perfect homology in synaptic circuitry between the VPMP and other sensory thalamic nuclei. More specifically, the inputs into other primary sensory thalamic nuclei (i.e., dLGN, vMGN, and VB—ventrobasal complex, which includes VPM and VPL) include driver inputs from primary sensory projections, inhibitory inputs from the TRN and the interneurons, and modulatory inputs from the brainstem and cortex. Each of these groups has been shown to display distinct morphological and molecular properties, and these will be addressed below.
Primary excitatory inputs to tree shrew VPMP
In the sensory thalamus, the input axons from their respective sensory organ bring the largest terminal boutons found in dLGN, MGN, or VB (Bartlett et al., 2000; Cavdar et al., 2011; Erisir et al., 1998; Jones & Rockel, 1971; Morest, 1975; Smith et al., 1964; Van Horn et al., 2000). The large terminals also constitute the VGLUT2 immunoreactive populations in all major primary sensory nuclei of the thalamus (Fremeau et al., 2001; Rovó et al., 2012; Varoqui et al., 2002). Furthermore, the afferent axon terminals from sensory organs to the thalamus tend to make multiple synaptic zones, synapse onto more proximal dendrites and engage in triads in glomeruli (Bartlett et al., 2000; Çavdar et al., 2011; Hamos et al., 1987; Jones & Rockel, 1971; McAlliser & Wells, 1981; Montero, 1991; Peschanski et al., 1985; Rovó et al., 2012). The large-sized terminal population found in the tree shrew VPMP have all of these morphological characteristics and also have large VGLUT2 positive boutons, which display many large mitochondria, asymmetric synapses and they provide a small portion of synapses in the nucleus. Overall, the properties exhibited by VGLUT2 positive terminals suggest that this population of terminals in the tree shrew VPMP are homologous to typical driver inputs seen in other sensory thalamic nuclei.
Modulatory inputs to the tree shrew VPMP
Modulatory inputs to thalamic nuclei create complex circuitry that affects the response of relay cells and the information they transmit to the cortex. Studies mostly in the dLGN, and at varying degrees in the MGN and VB, have characterized several modulatory inputs. These include medium-sized local GABAergic terminals providing feedback from inhibitory interneurons and the TRN, small-sized glutamatergic terminals with feedback from layer 6 of the cortex, and small-sized cholinergic terminals with inputs from the brainstem (Coomes et al., 2002; Erisir et al., 1997a,b; Hallanger et al., 1990; Hamos et al., 1985; Liu et al., 1995; Montero, 1986; Ohara et al., 1989; Ohara & Lieberman, 1993; Ojima, 1994; Parent & Descarries, 2008; Rovó et al., 2012). Corticothalamic terminals are small, target more distal regions of relay cell dendrites, and they are positive for VGLUT1. These terminals are also extra-glomerular and constitute the largest population of synapses in the sensory nuclei (Bartlett et al., 2000; Erisir et al., 1997a,b; Erisir et al., 1998; Liu et al., 1995; Ojima, 1994; Van Horn et al., 2000). Our results show that the smallest-sized terminals in the tree shrew VPMP are positive for VGLUT1, and these display the typical morphology of corticothalamic terminals from layer VI of the cortex.
The cholinergic axons into the thalamus originate in the PBN of the brain stem, and they provide both a fast and a slow excitatory input to relay cell dendrites (Erisir et al., 1997b; Rowell et al., 2003; McCormick, 1992; Plummer et al., 1999). In the dLGN of cats, these terminals act as modulators by altering the firing pattern of thalamic relay cells from burst mode to tonic mode (Lu et al., 1993). Cholinergic terminals have been described as small (although larger than terminals originating in the cortex) with round vesicles that contact the more proximal portions of relay cell dendrites compared to glutamatergic terminals from the cortex (Erisir et al., 1997b; Sherman & Guillery, 1998). Our results mirror these findings and show that the tree shrew VPMP possesses cholinergic terminals that contain round vesicles and that are larger than VGLUT1 terminals.
In the non-chemical sensory nuclei, inhibitory synapses originate from the axons and the dendritic appendages of the interneurons, and the axons of the TRN cells (Cucchiaro et al., 1991; Guillery, 1969b; Montero, 1986; Sherman & Guillery, 2001). The inhibitory terminals display sparse pleomorphic vesicles (ranging from flat to small round shape), symmetric synapses (i.e., with thin postsynaptic density), and may form axo-axonal, axo-dendritic, and dendro-dendritic synapses (Guillery, 1969b; Montero, 1986; Uhlrich & Cucchiaro, 1992). Terminal boutons that display these characteristics are also found in the tree shrew VPMP, suggesting the utilization of a full complement of inhibitory interactions that are typical for non-chemical sensory nuclei.
One of the most prominent ultrastructural characteristics seen in visual, auditory, and somatosensory thalamic nuclei are glomerular arrangements and triads. Triads consist of a driver input terminal that then contacts both an interneuron dendrite and a relay cell dendrite, with the interneuron dendrite also synapsing onto the same relay cell dendrite. Astrocytic processes can encapsulate one or more triads to form a glomerulus (Coomes et al., 2002; Famiglietti & Peters, 1972; Govindaiah & Cox, 2006; Jones & Powell, 1969; Sherman & Guillery, 2002). These structures likely provide inhibitory localized feedback onto relay cell dendrites and may regulate the timing involved in sensory transmission (Famiglietti & Peters, 1972; Hamos et al., 1987, 1985; Lam et al., 2005; Saul, 2008; reviewed in Sherman, 2004). The tree shrew VPMP also contains glomeruli and triads, suggesting that the coding and the modulation of taste information in the tree shrew VPMP may involve similar synaptic network properties as found in the other sensory thalamic nuclei.
The differences and similarities between tree shrew and primate VPMP
Although little is known about the ultrastructural characteristics of the primate taste thalamus, information on the chemical characteristics of primate VPMP as well as synaptic characteristics seen in other sensory thalamic nuclei (for example, dLGN and VPM) suggests close similarities between the primate and the tree shrew VPMP. Primates and tree shrews both possess a well-developed dLGN, pretectal nucleus, and dorsomedial nucleus as opposed to rodents (Campbell, 1966a, 1966b; Goldby, 1941). The cytoarchitectonic borders of the VPMP can be easily visualized in both primates and tree shrews with CO staining, indicating that this area in both species is highly metabolically active (Cerkevich et al., 2013; Durand et al., 2016; E. G. Jones et al., 1986; Saddoris et al., 2009; Wong-Riley & Norton, 1988). Like the tree shrew VPMP, inputs into primary sensory thalamic nuclei are positive for the glutamate transporter VGluT2 and are the largest terminals seen in these nuclei (Cerkevich et al., 2013; E. G. Jones, 1985; Kaas et al., 1972; Rovó et al., 2012; Sherman & Guillery, 2001). Finally, modulatory inputs into the primary sensory nuclei of the primate thalamus are the same as what have been characterized here in the tree shrew VPMP. These inputs include small, VGluT1 positive terminals originating from the cortex (Casagrande et al., 2006; Rovó et al., 2012; Sherman & Guillery, 2001), GABAergic interneurons (Casagrande et al., 2006; Ohara et al., 1989a; Sherman & Guillery, 2001; Y. Smith et al., 1987), and ChAT positive terminals from the brainstem (Martha E Bickford et al., 2000; Casagrande et al., 2006; Fitzpatrick & Diamond, 1980; Sherman & Guillery, 2001; Wilson et al., 1999). These similarities suggest that the flow of information through the primate and tree shrew thalamus, and more specifically the VPMP, are very similar, and these similarities position tree shrews to be a reliable model for taste processing through the thalamus.
Differences and similarities between tree shrew VPMP and rat VPMpc:
In direct contrast to its similarities to the thalamic nuclei of visual, auditory and somatosensory, that is non-chemical senses, the synaptic circuitry in the taste thalamus of the tree shrew is different from that in rats in several aspects: In a recent study, Holtz et al. (2015) placed tracer injections into the waist region of the PBN and in insular cortex in order to characterize the morphological properties of inputs into the rodent VPMpc. Their results showed that the parabrachiothalamic terminals were sparsely distributed in the VPMpc, and they appeared as very large boutons filled with peptide releasing vesicles, which selectively wrap around the dendrites as they emerge from the neuron soma and make a large number of synaptic zones. This contrasts with the current findings in the tree shrew VPMP, where the large terminals are distributed along the dendrite segments and make relatively fewer synaptic zones. The rat VPMpc also lacks GABAergic interneurons, or triadic arrangements involving presynaptic and postsynaptic dendrites. Thus, a primary motif for synaptic interactions among primary input axons, inhibitory inputs and the thalamocortical cells (Arcelli et al., 1997; Barbaresi et al., 1986; Govindaiah & Cox, 2006), is found in tree shrew taste thalamus but not in the rat taste thalamus (Figure 8).
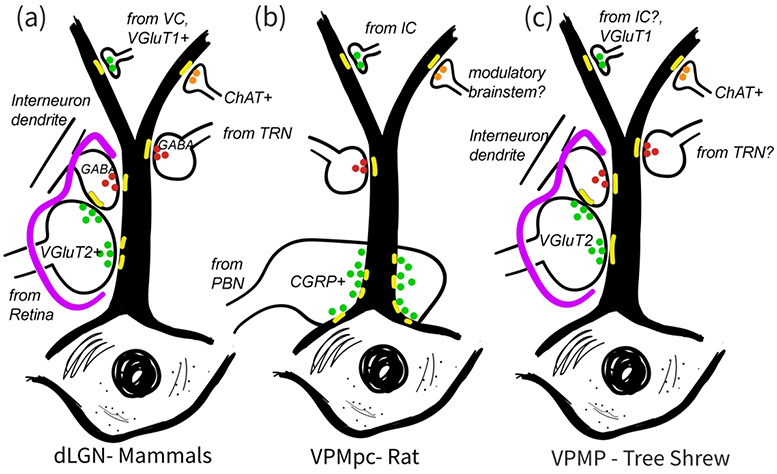
Figure 8:
Schematic comparison of synaptic inputs onto relay cell dendrites in the dLGN of various mammals (a), in the VPMpc, thalamic taste nucleus of the rat (b), and in the VPMP, taste nucleus of the tree shrew (c). A primary characteristics of the mammalian dLGN circuitry, that is the glia (purple) encased glomerular triads involving primary sensory inputs and pre-synaptic interneuron dendrites, is a common feature of the tree shrew VPMP, but they are not found in the rat VPMpc. Instead, the axons that bring the primary sensory axons to rat VPMP target soma and dendrite emergence regions and do not engage in triads. While large-sized glutamatergic terminals both in mammalian dLGN and the tree shrew VPMP contain VGluT2, the rat VPMpc does not display any prominent VGluT2 label. Instead, the large terminals in rat VPMpc uniquely contain CGRP peptide. Similar to mammalian dLGN and unlike rat VPMpc, tree shrew VPMP contains GABAergic cells. Also similar to in mammalian dLGN, the tree shrew VPMP prominently contains ChAT+ terminals. In all three structures, the small-size terminals including those from primary visual cortex or the insular cortex, target small caliber, distal dendrites, and these are VGluT1+ in the mammalian dLGN and the tree shrew VPMP.
What do the differences in rat and tree shrew taste thalamus synaptic circuitries indicate?
Since the earlier studies five decades ago, it has been known that the taste pathway in rodents is organized differently than that in primates. In primates, the VPMP, the primary thalamic nucleus that transmits taste information to the insular cortex for higher-order processing (Benjamin & Burton, 1968; T C Pritchard et al., 1989), receives its main excitatory input from the rostral NTS, which receives taste afferents originating from the taste buds (Beckstead et al., 1980; Hamilton & Norgren, 1984). However, in the rodent gustatory system, the flow of taste information from tongue to cortex takes a detour: Instead of a direct connection to the taste thalamus as in primates, the NTS taste neurons project to the parabrachial nucleus of the pons (PBN) (Norgren, 1976; Norgren & Leonard, 1971). From the PBN, gustatory information splits into two pathways. One projection forms the ventral forebrain pathway of the PBN, carrying taste-related affective information to limbic structures including the amygdala and lateral hypothalamus (Bester et al., 1997; Jhamandas et al., 1996; Norgren, 1976; Norgren & Leonard, 1971; Saper & Loewy, 1980). The second pathway originates in the waist region of the PBN and sends taste information through strong excitatory signals to the VPMpc (Herbert et al., 1990; Norgren & Pfaffmann, 1975; Saper & Loewy, 1980; Tokita et al., 2010). Both the VPMpc and the amygdala have reciprocal connections with the Insular Cortex and each other (Holtz et al., 2015; Kosar et al., 1986; Maffei et al., 2012; Nakashima et al., 2000; Norgren, 1976; Tokita et al., 2010). In contrast, the taste neurons of the primate NTS do not project to the PBN; there are also no axonal connections between the waist PBN and the taste region of the thalamus (Pritchard et al., 2000). Side-by-side comparisons of rodent and primate gustatory systems often downplay the anatomical differences and rely on the similarities of the behavioral measures that are attributed to the thalamus or amygdala function (Pritchard & Di Lorenzo, 2015). The current study provides novel anatomical evidence to underscore crucial differences between primate-like species and the rats regarding the role of their thalamus in cortical function, and it suggests caution for generalizing findings of rodent taste thalamus function to those in non-human primates and humans. Furthermore, the findings that reveal the similarities between the taste and other sensory thalamic nuclei may now allow the opportunity to apply an extensive, accumulated knowledgebase on visual, somatosensory, and auditory nuclei in understanding the principles of taste sense processing through the thalamus.
ACKNOWLEDGMENTS
We acknowledge the electron microscopy technical expertise by Bonnie Sheppard.
Funded by: NIH-NIDCD R01DC10183 to AE; UVA College Council Minerva Award to MP; NIH R01EY016155 to HP.
Abbreviations:
- ChAT
choline acetyltransferase
- CO
cytochrome oxidase
- DAB
diaminobenzidine
- dLGN
dorsal lateral geniculate nucleus
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase
- LGN
lateral geniculate nucleus
- MGN
medial geniculate nucleus
- NR
red nucleus
- NTS
nucleus of the solitary tract
- OT
optic tract
- PBN
parabrachial nucleus of the pons
- PF
parafascicular nucleus of the thalamus
- PUL
pulvinar nucleus
- SC
superior colliculus
- SN
substantia nigra
- TRN
thalamic reticular nucleus
- VB
ventrobasal complex
- VGLUT1
vesicular glutamate transporter type 1
- VGLUT2
vesicular glutamate transporter type 2
- VPI
ventral posterior inferior nucleus
- VPL
ventral posterolateral nucleus
- VPMP
ventral posteromedial parvocellular nucleus
- VPMpc
ventral posteromedial parvocellular nucleus
- VPM
ventral posteromedial nucleus
Footnotes
Competing Interests: The authors declare that they have no competing interests
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agarwala S, Güniük AE, May JG, & Petry HM (1992). Immunohistochemical organization of the ventral lateral geniculate nucleus in the tree shrew. Journal of Comparative Neurology, 318(3), 267–276. 10.1002/cne.903180304 [DOI] [PubMed] [Google Scholar]
- Agca S, Houen G, & Trier NH (2014). Characterization of continuous B-cell epitopes in the N-terminus of glutamate decarboxylase67 using monoclonal antibodies. Journal of Peptide Science, 20(12), 928–934. 10.1002/psc.2703 [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, & Cechetto DF (1991). Organization of visceral and limbic connections in the insular cortex of the rat. Journal of Comparative Neurology, 311(1), 1–16. 10.1002/cne.903110102 [DOI] [PubMed] [Google Scholar]
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, & Spreafico R (1997). GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Research Bulletin, 42(1), 27–37. 10.1016/S0361-9230(96)00107-4 [DOI] [PubMed] [Google Scholar]
- Balaram P, Isaamullah M, Petry HM, Bickford ME, & Kaas JH (2015). Distributions of vesicular glutamate transporters 1 and 2 in the visual system of tree shrews (Tupaia belangeri). Journal of Comparative Neurology, 523(12), 1792–1808. 10.1002/cne.23727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Wei H, Reed JL, Bickford ME, Petry HM, & Kaas JH (2013). Cortical projections to the superior colliculus in tree shrews (Tupaia belangeri). The Journal of Comparative Neurology, 521(7), 1614–1632. 10.1002/cne.23249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, & Rustioni A (1986). GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Research, 382(2), 305–326. 10.1016/0006-8993(86)91340-5 [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, & Smith PH (2000). Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience, 100(4), 811–828. 10.1016/S0306-4522(00)00340-7 [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Morse JR, & Norgren R (1980). The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. The Journal of Comparative Neurology, 190(2), 259–282. 10.1002/cne.901900205 [DOI] [PubMed] [Google Scholar]
- Benjamin RM, & Burton H (1968). Projection of taste nerve afferents to anterior opercular- insular cortex in squirrel monkey (Saimiri sciureus). Brain Research, 7(2), 221–231. 10.1016/0006-8993(68)90100-5 [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, & Bernard JF (1997). Organization of efferent projections from the parabrachial area to the hypothalamus: A Phaseolus vulgaris-leucoagglutinin study in the rat. Journal of Comparative Neurology, 383(3), 245–281. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, & Guido W (2010). Synaptic development of the mouse dorsal lateral geniculate nucleus. The Journal of Comparative Neurology, 518(5), 622–635. 10.1002/cne.22223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford Martha E. (2015). Thalamic circuit diversity: Modulation of the driver/modulator framework. Frontiers in Neural Circuits, 9(January2016), 1–8. 10.3389/fncir.2015.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford Martha E. (2019). Synaptic organization of the dorsal lateral geniculate nucleus. European Journal of Neuroscience, 49(7), 938–947. 10.1111/ejn.13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Ramcharan E, Godwin DW, Erişir A, Gnadt J, & Sherman SM (2000). Neurotransmitters contained in the subcortical extraretinal inputs to the monkey lateral geniculate nucleus. Journal of Comparative Neurology, 424(4), 701–717. [DOI] [PubMed] [Google Scholar]
- Blomquist AJ, Benjamin RM, & Emmers R (1962). Thalamic localization of afferents from the tongue in squirrel monkey (Saimiri sciureus). Journal of Comparative Neurology, 118(1), 77–87. 10.1002/cne.901180106 [DOI] [PubMed] [Google Scholar]
- Campbell CBG (1966a). Taxonomic status of Tree Shrews. Science, 153. [DOI] [PubMed] [Google Scholar]
- Campbell CBG (1966b). The Relationships of the Tree Shrews: The Evidence of the Nervous System. Evolution, 20(3), 276–281. [DOI] [PubMed] [Google Scholar]
- Cao J, Yang E. Bin, Su J. J., Li, Y., & Chow P (2003). The tree shrews: Adjuncts and alternatives to primates as models for biomedical research. Journal of Medical Primatology, 32(3), 123–130. 10.1034/j.1600-0684.2003.00022.x [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Royal DW, & Sáry G (2006). Extraretinal Inputs and Feedback Mechanisms to the Lateral Geniculate Nucleus (LGN). In The Primate Visual System: A Comparative Approach (pp. 191–211). 10.1002/0470868112.ch7 [DOI] [Google Scholar]
- Cavdar S, Hacioǧlu H, Şirvanci S, Keskinöz E, & Onat F (2011). Synaptic organization of the rat thalamus: A quantitative study. Neurological Sciences, 32(6), 1047–1056. 10.1007/s10072-011-0606-4 [DOI] [PubMed] [Google Scholar]
- Cechetto DF, & Saper CB (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. Journal of Comparative Neurology, 262(1), 27–45. 10.1002/cne.902620104 [DOI] [PubMed] [Google Scholar]
- Cerkevich CM, Qi HX, & Kaas JH (2013). Thalamic input to representations of the teeth, tongue, and face in somatosensory area 3b of macaque monkeys. Journal of Comparative Neurology, 521(17), 3954–3971. 10.1002/cne.23386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clascá F, Llamas A, & Reinoso-Suárez F (1997). Insular cortex and neighboring fields in the cat: A redefinition based on cortical microarchitecture and connections with the thalamus. Journal of Comparative Neurology, 384(3), 456–482. [DOI] [PubMed] [Google Scholar]
- Colonnier M, & Guillery RW (1964). Synaptic organization in the lateral geniculate nucleus of the monkey. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 62(3), 333–355. [DOI] [PubMed] [Google Scholar]
- Coomes DL, Bickford ME, & Schofield BR (2002). GABAergic circuitry in the dorsal division of the cat medial geniculate nucleus. The Journal of Comparative Neurology, 453(1), 45–56. 10.1002/cne.10387 [DOI] [PubMed] [Google Scholar]
- Corson JA, Aldridge A, Wilmoth K, & Erisir A (2012). A survey of oral cavity afferents to the rat nucleus tractus solitarii. Journal of Comparative Neurology, 520(3), 495–527. 10.1002/cne.22715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Cucchiaro JB, Uhlrich DJ, & Sherman SM (1991). Electron-microscopic analysis of synaptic input from the perigeniculate nucleus to the A-laminae of the lateral geniculate nucleus in cats. Journal of Comparative Neurology, 310(3), 316–336. 10.1002/cne.903100304 [DOI] [PubMed] [Google Scholar]
- Day-Brown JD, Slusarczyk AS, Zhou N, Quiggins R, Petry HM, & Bickford ME (2017). Synaptic organization of striate cortex projections in the tree shrew: A comparison of the claustrum and dorsal thalamus. Journal of Comparative Neurology, 525(6), 1403–1420. 10.1002/cne.23998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Iyer R, Mizuseki K, De Vries S, Mihalas S, & Reid RC (2016). A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. Journal of Neuroscience, 36(48), 12144–12156. 10.1523/JNEUROSCI.1741-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmers R (1966). Separate Relays of Tactile, Pressure, Thermal, and Gustatory Modalities in the Gat Thalamus. Proceedings of the Society for Experimental Biology and Medicine, 121(2), 527–531. [DOI] [PubMed] [Google Scholar]
- Emmers Raimond, Benjamin RM, & Blomquist AJ (1962). Thalamic localization of afferents from the tongue in albino rat. Journal of Comparative Neurology, 118(1), 43–48. 10.1002/cne.901180104 [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, & Sherman SM (1997). Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: A comparison with corticogeniculate terminals. The Journal of Comparative Neurology, 377(4), 535–549. [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, & Sherman SM (1997). Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1517–1520. 10.1073/pnas.94.4.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, & Sherman SM (1998). Distribution of synapses in the lateral geniculate nucleus of the cat: Differences between laminae A and A1 and between relay cells and interneurons. The Journal of Comparative Neurology, 390(2), 247–255. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, & Peters A (1972). The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. Journal of Comparative Neurology, 144(3), 285–333. 10.1002/cne.901440304 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D (1996). The functional organization of local circuits in visual cortex: Insights from the study of tree shrew striate cortex. Cerebral Cortex, 6(3), 329–341. 10.1093/cercor/6.3.329 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, & Diamond IT (1980). Distribution of acetylcholinesterase in the geniculo striate system of Galago senegalensis and Aotus trivirgatus: Evidence for the origin of the reaction product in the lateral geniculate body. Journal of Comparative Neurology, 194(4), 703–719. 10.1002/cne.901940402 [DOI] [PubMed] [Google Scholar]
- Fremeau RT Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, & Edwards RH (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron, 31(2), 247–260. 10.1016/S0896-6273(01)00344-0 [DOI] [PubMed] [Google Scholar]
- Goldby F (1941). The normal histology of the thalamus in the phalanger, Trichosurus vulpecula. Journal of Anatomy, 75(Pt 2), 197–224.3. [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, & Cox CL (2006). Excitatory actions of synaptically released catecholamines in the rat lateral geniculate nucleus. Neuroscience, 137(2), 671–683. 10.1016/j.neuroscience.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Guillery RW (1969a). A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 96(1), 39–48. [DOI] [PubMed] [Google Scholar]
- Guillery RW (1969b). The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 96(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Guillery RW, & Sherman SM (2002). Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron, 33(2), 163–175. 10.1016/S0896-6273(01)00582-7 [DOI] [PubMed] [Google Scholar]
- Hajdu F, Hassler R, Somogyi G. (1982). Neuronal and synaptic organization of the lateral geniculate nucleus of the tree shrew, Tupaia glis. Cell Tissue Res. 224(1):207–223. doi: 10.1007/BF00217280 [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Price SD, Lee HJ, Steininger TL, & Wainer BH (1990). Ultrastructure of cholinergic synaptic terminals in the thalamic anteroventral, ventroposterior, and dorsal lateral geniculate nuclei of the rat. Journal of Comparative Neurology, 299(4), 482–492. 10.1002/cne.902990408 [DOI] [PubMed] [Google Scholar]
- Hamilton RB, & Norgren R (1984). Central projections of gustatory nerves in the rat. Journal of Comparative Neurology, 222(4), 560–577. 10.1002/cne.902220408 [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, & Sherman SM (1987). Synaptic circuits involving an individual retinogeniculate axon in the cat. Journal of Comparative Neurology, 259(2), 165–192. 10.1002/cne.902590202 [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, & Sherman SM (1985). Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature, 317(6038), 618–621. 10.1038/317618a0 [DOI] [PubMed] [Google Scholar]
- Hannig S, & Jürgens U (2006). Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Experimental Brain Research, 169(1), 92–105. 10.1007/S00221-005-0128-5 [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, & Saper CB (1990). Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. Journal of Comparative Neurology, 293(4), 540–580. 10.1002/cne.902930404 [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, & El Mestikawy S (2001). The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(22), 2–7. 10.1523/jneurosci.21-22-j0001.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Norton TT, & R RM (1988). Laminar organization and ultrastructure of GABA-immunoreactive neurons and processes in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia belangeri). Visual Neuroscience, 1(2), 189–204. [DOI] [PubMed] [Google Scholar]
- Holtz SL, Fu A, Loflin W, Corson JA, & Erisir A (2015). Morphology and connectivity of parabrachial and cortical inputs to gustatory thalamus in rats. Journal of Comparative Neurology, 523(1), 139–161. 10.1002/cne.23673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Qi H-X, Jain N, & Kaas JH (2007). Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of new world monkeys. The Journal of Comparative Neurology, 501(1), 95–120. 10.1002/cne.21232 [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Petrov T, Harris KH, Vu T, & Krukoff TL (1996). Parabrachial nucleus projection to the amygdala in the rat: Electrophysiological and anatomical observations. Brain Research Bulletin, 39(2), 115–126. 10.1016/0361-9230(95)02084-5 [DOI] [PubMed] [Google Scholar]
- Jones EG (1985). The Thalamus. Springer Science & Business Media. [Google Scholar]
- Jones EG, Hendry SHC, & Brandon C (1986). Cytochrome oxidase staining reveals functional organization of monkey somatosensory thalamus. Experimental Brain Research, 62(2), 438–442. [DOI] [PubMed] [Google Scholar]
- Jones EG, & Rockel AJ (1971). The synaptic organization in the medial geniculate body of afferent fibres ascending from the inferior colliculus. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 113(1), 44–66. 10.1007/BF00331201 [DOI] [PubMed] [Google Scholar]
- Jones Edward G., & Powell TP (1969). Electron microscopy of synaptic glomeruli in the thalamic relay nuclei of the cat. Proceedings of the Royal Society of London. Series B. Biological Sciences, 172(27), 153–171. 10.1098/rspb.1969.0017 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Guillery RW, & Allman JM (1972). Some principles of organization in the dorsal lateral geniculate nucleus. Brain, Behavior and Evolution, 6(1), 253–299. 10.1159/000123715 [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, & Norgren R (1986). Gustatory cortex in the rat. II. Thalamocortical projections. Brain Research, 379(2), 342–352. 10.1016/0006-8993(86)90788-2 [DOI] [PubMed] [Google Scholar]
- Lam Y, Cox CL, Varela C, & Sherman SM (2005). Morphological correlates of triadic circuitry in the lateral geniculate nucleus of cats and rats. Journal of Neurophysiology, 93(2), 748–757. 10.1152/jn.00256.2004 [DOI] [PubMed] [Google Scholar]
- Land PW, Kyonka E, & Shamalla-Hannah L (2004). Vesicular glutamate transporters in the lateral geniculate nucleus: Expression of VGLUT2 by retinal terminals. Brain Research, 996(2), 251–254. 10.1016/j.brainres.2003.10.032 [DOI] [PubMed] [Google Scholar]
- Lieberman AR, & Webster KE (1974). Aspects of the synaptic organization of intrinsic neurons in the dorsal lateral geniculate nucleus. Journal of Neurosytology, 3(6), 677–710. [DOI] [PubMed] [Google Scholar]
- Lin C-S, Merzenich MM, Sur M, & Kaas JH (1979). Connections of areas 3b and 1 of the parietal somatosensory strip with the ventroposterior nucleus in the owl monkey Aotus trivirgatus. Journal of Comparative Neurology, 185(2), 355–371. 10.1002/cne.901850209 [DOI] [PubMed] [Google Scholar]
- Liu XB, Honda CN, & Jones EG (1995). Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. The Journal of Comparative Neurology, 352(1), 69–91. 10.1002/cne.903520106 [DOI] [PubMed] [Google Scholar]
- Ma W, Peschanski M, & Ralston HJ (1987). Fine structure of the spinothalamic projections to the central lateral nucleus of the rat thalamus. Brain Research, 414(1), 187–191. 10.1016/0006-8993(87)91345-X [DOI] [PubMed] [Google Scholar]
- Maffei A, Haley M, & Fontanini A (2012). Neural processing of gustatory information in insular circuits. Current Opinion in Neurobiology, 22(4), 709–716. 10.1016/j.conb.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha SL, Shantha TR, & Bourne GH (1967). Histochemical mapping of the distribution of monoamine oxidase in the diencephalon and basal telencephalic centers of the brain of squirrel monkey (Saimiri sciureus). Brain Research, 6, 570–586. 10.1136/bmj.3.5565.607 [DOI] [PubMed] [Google Scholar]
- McAllister JP, & Wells J (1981). The structural organization of the ventral posterolateral nucleus in the rat. The Journal of Comparative Neurology, 197(2), 271–301. 10.1002/cne.901970208 [DOI] [PubMed] [Google Scholar]
- McCormick DA (1992). Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology, 39(4), 337–388. 10.1016/0301-0082(92)90012-4 [DOI] [PubMed] [Google Scholar]
- McCormick DA, & Prince DA (1987). Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. The Journal of Physiology, 392(1), 147–165. 10.1113/jphysiol.1987.sp016774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM (1986). Localization of γ-aminobutyric acid (GABA) in type 3 cells and demonstration of their source to f2 terminals in the cat lateral geniculate nucleus: A golgi-electron-microscopic GABA-immunocytochemical study. Journal of Comparative Neurology, 254(2), 228–245. 10.1002/cne.902540207 [DOI] [PubMed] [Google Scholar]
- Montero VM (1991). A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Experimental Brain Research, 86(2), 257–270. [DOI] [PubMed] [Google Scholar]
- Morest DK (1975). Synaptic relationships of Golgi type II cells in the medial geniculate body of the cat. Journal of Comparative Neurology, 162(2), 157–193. 10.1002/cne.901620202 [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Eizirik E, O’Brien SJ, Madsen O, Scally M, Douady CJ, Teeling E, Ryder OA, Stanhope MJ, De Jong WW, & Springer MS (2001). Resolution of the early placental mammal radiation using bayesian phylogenetics. Science, 294(5550), 2348–2351. 10.1126/science.1067179 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, & Männistö PT (2008). Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. Journal of Comparative Neurology, 507(5), 1694–1708. 10.1002/cne.21642 [DOI] [PubMed] [Google Scholar]
- Nakashima M, Uemura M, Yasui Y, Ozaki HS, Tabata S, Taen A, Yasui K, Ozaki HS, Tabata S, & Taen A (2000). An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: Distribution of neurons projecting to the insular cortex and amygdaloid complex. Neuroscience Research, 36(4), 297–309. 10.1016/S0168-0102(99)00129-7 [DOI] [PubMed] [Google Scholar]
- Nei M, & Glazko GV (2002). Estimation of divergence times for a few mammalian and several primate species. Journal of Heredity, 93(3), 157–164. 10.1093/jhered/93.3.157 [DOI] [PubMed] [Google Scholar]
- Norgren R (1976). Taste pathways to hypothalamus and amygdala. The Journal of Comparative Neurology, 166(1), 17–30. 10.1002/cne.901660103 [DOI] [PubMed] [Google Scholar]
- Norgren R, & Leonard CM (1971). Taste pathways in the rat brainstem. Science, 173(4002), 1136–1139. [DOI] [PubMed] [Google Scholar]
- Norgren R, & Pfaffmann C (1975). The pontine taste area in the rat. Brain Research, 91(1), 99–117. 10.1016/0006-8993(75)90469-2 [DOI] [PubMed] [Google Scholar]
- Ohara PT, Chazal G, & Ralston HJ (1989). Ultrastructural analysis of gaba-immunoreactive elements in the monkey thalamic ventrobasal complex. Journal of Comparative Neurology, 283(4), 541–558. 10.1002/cne.902830408 [DOI] [PubMed] [Google Scholar]
- Ohara PT, & Lieberman AR (1993). Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. Journal of Neurocytology, 22(9), 815–825. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8270964&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- Ojima H (1994). Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cerebral Cortex, 4(6), 646–663. 10.1093/cercor/4.6.646 [DOI] [PubMed] [Google Scholar]
- Parent M, & Descarries L (2008). Acetylcholine innervation of the adult rat thalamus: Distribution and ultrastructural features in dorsolateral geniculate, parafascicular, and reticular thalamic nuclei. Journal of Comparative Neurology, 511(5), 678–691. 10.1002/cne.21868 [DOI] [PubMed] [Google Scholar]
- Persson S, Boulland JL, Aspling M, Larsson M, Fremeau RT Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA, & Broman J (2006). Distribution of Vesicular Glutamate Transporters 1 and 2 in the Rat Spinal Cord, with a Note on the Spinocervical Tract. Journal of Comparative Neurology, 497, 683–701. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Roudier F, Ralston HJ, & Besson JM (1985). Ultrastructural analysis of the terminals of various somatosensory pathways in the ventrobasal complex of the rat thalamus: An electron-microscopic study using wheatgerm agglutinin conjugated to horseradish peroxidase as an axonal tracer. Somatosensory & Motor Research, 3(1), 75–87. 10.3109/07367228509144578 [DOI] [PubMed] [Google Scholar]
- Petry HM, & Bickford ME (2019). The Second Visual System of The Tree Shrew. Journal of Comparative Neurology, 527(3), 679–693. 10.1002/cne.24413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer KL, Manning KA, Levey AI, Rees HD, & Uhlrich DJ (1999). Muscarinic receptor subtypes in the lateral geniculate nucleus: A light and electron microscopic analysis. Journal of Comparative Neurology, 404(3), 408–425. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, & Norgren R (1989). Neural coding of gustatory information in the thalamus of Macaca mulatta. Journal of Neurophysiology, 61(1), 1–14. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=2918337&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- Pritchard Thomas C., & Di Lorenzo PM (2015). Central Taste Anatomy and Physiology of Rodents and Primates. Handbook of Olfaction and Gustation: Third Edition, 701–726. 10.1002/9781118971758.ch32 [DOI] [Google Scholar]
- Pritchard Thomas C., Hamilton RB, & Norgren R (2000). Projections of the parabrachial nucleus in the Old World monkey. Experimental Neurology, 165(1), 101–117. 10.1006/exnr.2000.7450 [DOI] [PubMed] [Google Scholar]
- Ranc V, Petruzziello F, Kretz R, Argandoña EG, Zhang X, & Rainer G (2012). Broad characterization of endogenous peptides in the tree shrew visual system. Journal of Proteomics, 75(9), 2526–2535. 10.1016/j.jprot.2012.01.028 [DOI] [PubMed] [Google Scholar]
- Rice MW, Roberts RC, Melendez-Ferro M, & Perez-Costas E (2011). Neurochemical characterization of the tree shrew dorsal striatum. Frontiers in Neuroanatomy, 5(August), 1–17. 10.3389/fnana.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovó Z, Ulbert I, & Acsády L (2012). Drivers of the Primate Thalamus. Journal of Neuroscience, 32(49), 17894–17908. 10.1523/JNEUROSCI.2815-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Holland PC, & Gallagher M (2009). Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. Journal of Neuroscience, 29(49), 15386–15396. 10.1523/JNEUROSCI.3233-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, & Loewy AD (1980). Efferent connections of the parabrachial nucleus in the rat. Brain Research, 197(2), 291–317. 10.1016/0006-8993(80)91117-8 [DOI] [PubMed] [Google Scholar]
- Saul AB (2008). Lagged cells in alert monkey lateral geniculate nucleus. Visual Neuroscience, 25(5–6), 647–659. 10.1017/S0952523808080784 [DOI] [PubMed] [Google Scholar]
- Sherman SM (2004). Interneurons and triadic circuitry of the thalamus. Trends in Neurosciences, 27(11), 670–675. 10.1016/j.tins.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Sherman SM (2007). The thalamus is more than just a relay. Current Opinion in Neurobiology, 17(4), 417–422. 10.1016/j.conb.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, & Guillery RW (1996). Functional organization of thalamocortical relays. Journal of Neurophysiology, 76(3), 1367–1395. 10.1152/jn.1996.76.3.1367 [DOI] [PubMed] [Google Scholar]
- Sherman SM, & Guillery RW (1998). On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators.” Proceedings of the National Academy of Sciences of the United States of America, 95(12), 7121–7126. 10.1073/pnas.95.12.7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, & Guillery RW (2001). Exploring the Thalamus. Academic Pr. http://books.google.co.uk/books?id=L7WAeCvWJ04C&pg=PA54&dq=thalamus+jones&cd=3&source=gbs_api [Google Scholar]
- Sherman SM, & Guillery RW (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 357(1428), 1695–1708. 10.1098/rstb.2002.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, & Cassell MD (1998). Connections of the Anterior and Posterior Insular Cortices. Journal of Comparative Neurology, 468(May), 440–468. [DOI] [PubMed] [Google Scholar]
- Smith JM, O’Leary JL, Harris AB, & Gay AJ (1964). Ultrastructural features of the lateral geniculate nucleus of the cat. Journal of Comparative Neurology, 123(3), 357–377. 10.1002/cne.901230305 [DOI] [PubMed] [Google Scholar]
- Smith Y, Gubla PS, Parent A, Recherche D, De, Midecine C & De Montrcal U. (1987). Distribution of Gaba-Immunoreactive Neurons (Saimri Sciureus). 22(2), 579–591. [DOI] [PubMed] [Google Scholar]
- Tabor KM, Wong ROL, & Rubel EW (2011). Topography and morphology of the inhibitory projection from superior olivary nucleus to nucleus laminaris in chickens (Gallus gallus). Journal of Comparative Neurology, 519(2), 358–375. 10.1002/cne.22523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, & Shantha TR (1969). Stereotaxic brain atlas of the tree shrew (Tupaia glis). [Google Scholar]
- Tokita K, Inoue T, & Boughter JD (2010). Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience, 171(1), 351–365. 10.1016/j.neuroscience.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlrich DJ, & Cucchiaro JB (1992). GABAergic circuits in the lateral geniculate nucleus of the cat. Progress in Brain Research, 90, 171–192. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Roy A, Rhodes HJ, Culp JH, & Fitzpatrick D (2013). Transformation of receptive field properties from lateral geniculate nucleus to superficial V1 in the tree shrew. Journal of Neuroscience, 33(28), 11494–11505. 10.1523/JNEUROSCI.1464-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn SC, Erişir A, & Sherman SM (2000). Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. Journal of Comparative Neurology, 416(4), 509–520. [DOI] [PubMed] [Google Scholar]
- Vanni MP, Thomas S, Petry HM, Bickford ME, & Casanova C (2015). Spatiotemporal profile of voltage-sensitive dye responses in the visual cortex of tree shrews evoked by electric microstimulation of the dorsal lateral geniculate and pulvinar nuclei. Journal of Neuroscience, 35(34), 11891–11896. 10.1523/JNEUROSCI.0717-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MKH, Zhu H, Weihe E, & Erickson JD (2002). Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. Journal of Neuroscience, 22(1), 142–155. 10.1523/JNEUROSCI.22-01-00142.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Eisenback M, Datskovskaia A, Boyce M, & Bickford ME (2002). GABAergic pretectal terminals contact GABAergic interneurons in the cat dorsal lateral geniculate nucleus. 323, 141–145. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Manning KA, Forestner DM, Counts SE, & Uhlrich DJ (1999). Comparison of cholinergic and histaminergic axons in the lateral geniculate complex of the macaque monkey. Anatomical Record, 255(3), 295–305. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, & Norton TT (1988). Histochemical localization of cytochrome oxidase activity in the visual system of the tree shrew: Normal patterns and the effect of retinal impulse blockage. Journal of Comparative Neurology, 272(4), 562–578. 10.1002/cne.902720409 [DOI] [PubMed] [Google Scholar]
- Wong P, & Kaas JH (2009). Architectonic subdivisions of neocortex in the tree shrew (Tupaia belangeri). Anatomical Record. 292(7), 994–1027. 10.1002/ar.20916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Itoh K, Mizuno N, Nomura S, Takada M, Konishi A, & Kudo M (1983). The posteromedial ventral nucleus of the thalamus (VPM) of the cat: Direct ascending projections to the cytoarchitectonic subdivisions. Journal of Comparative Neurology, 220(2), 219–228. 10.1002/cne.902200209 [DOI] [PubMed] [Google Scholar]
- Zelano J, Berg A, Thams S, Hailer NP, & Cullheim S (2009). SynCAM1 expression correlates with restoration of central synapses on spinal motoneurons after two different models of peripheral nerve injury. Journal of Comparative Neurology, 517(5), 670–682. 10.1002/cne.22186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.