Figure 2. Futility Analysis, Biomarker Adaptation, and Study Analysis for an Intervention in PrecISE.
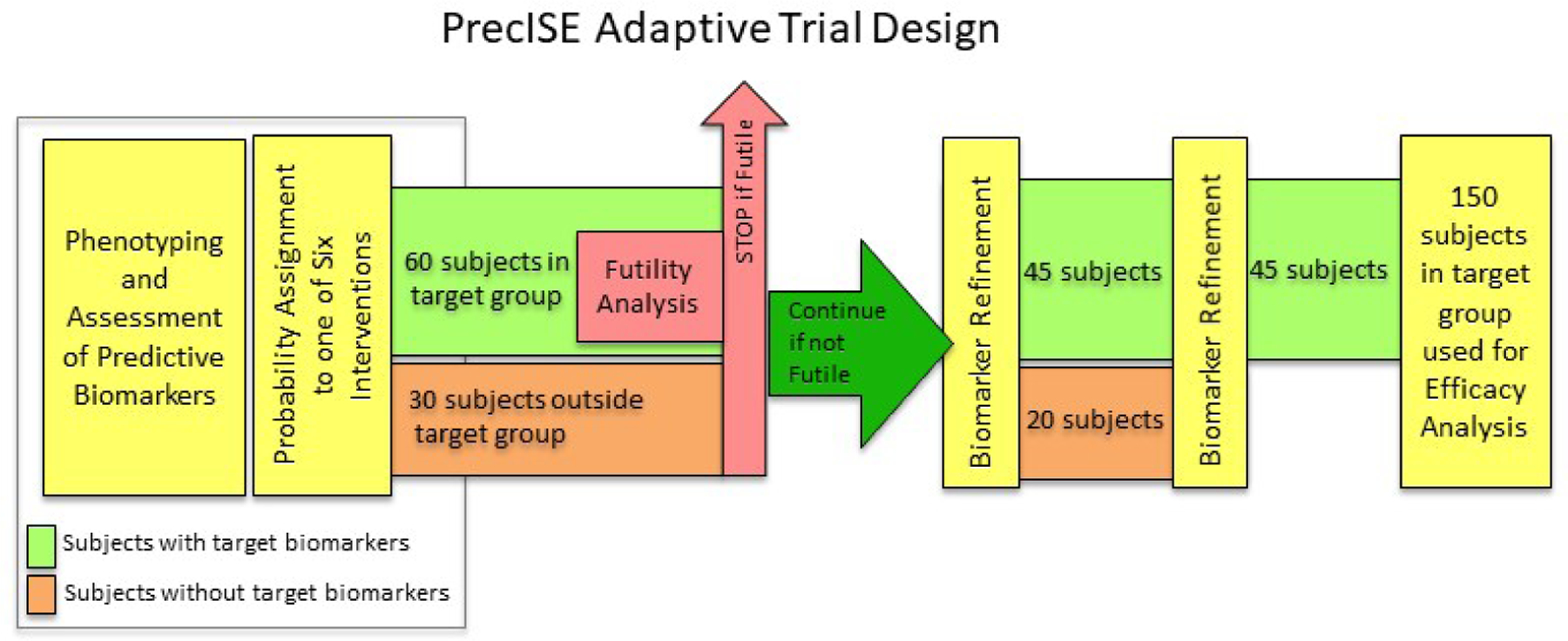
Patients are characterized, and their biomarker characteristics are determined and assigned during the run-in (see text). Based on these biomarker characteristics (Table II) they are preferentially (but not exclusively) assigned to interventions and placebos throughout their participation in the trial (Figure 1). As seen in Figure 2, patients within the target biomarker subgroup (green band) and those outside the target subgroup (orange band) are assigned to each intervention. After an intervention has accumulated 60 patients within the target biomarker subgroup (green band) and 30 subjects outside the biomarker subgroup (orange band), a futility analysis is performed restricted to the within-target 60 patients (see text). If the intervention is dropped for futility, all patients still receiving that intervention are assigned to alternative interventions. If the intervention is not dropped for futility, subgroups are refined and subjects within and outside the newly refined biomarker thresholds are enrolled and evaluated as outlined with an additional scheduled refinement as shown. When 150 patients are enrolled within the varying refined subgroups a final efficacy analysis is performed restricted to the 150 patients within the refined subgroups.
