Figure 3. Aggregation of TDP-43 impairs lysosome-autophagy function.
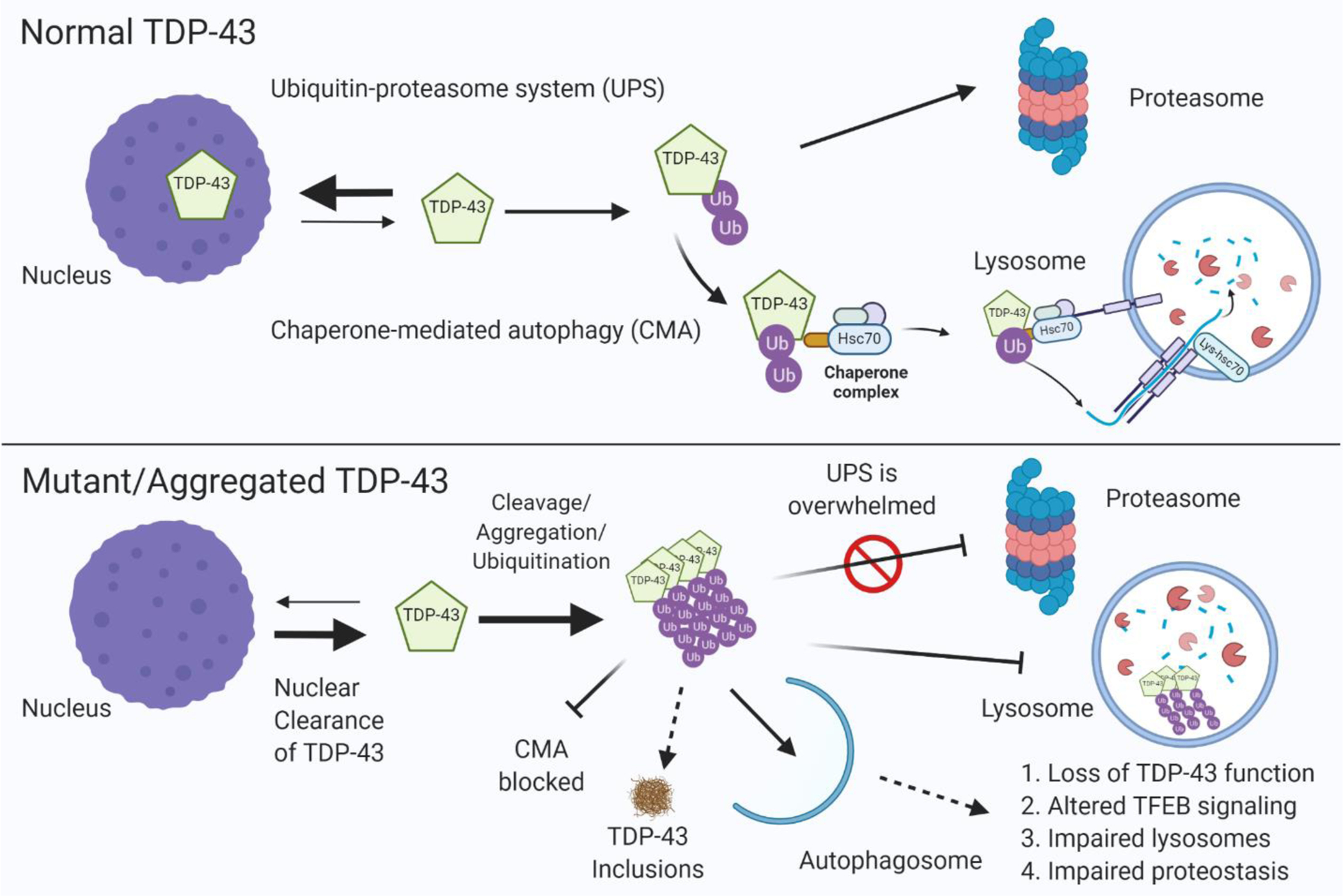
(Upper panel) In healthy cells, optimum levels of TDP-43 are maintained by 1) degradation through the ubiquitin–proteasome system (UPS) or 2) degradation through chaperone mediated autophagy (CMA) by binding to Hsc70 and import into the lysosome through LAMP2A. (Lower panel) TARDBP gene mutations or other causes (i.e. lysosome dysfunction cause by GRN or C9orf72 mutations) lead to accumulation and aggregation of TDP-43, which ultimately form insoluble inclusions. TDP-43 aggregates are not efficiently cleared by the proteasome which leads to their clearance through autophagosomes that required fusion with lysosomes. TDP-43 aggregates can block CMA and impair lysosome function, leading to a feed-forward toxic mechanism causing loss of TDP-43 function, TDP-43 inclusion formation, lysosome dysfunction, impaired protein homeostasis in the cell, and ultimately neurodegeneration.
