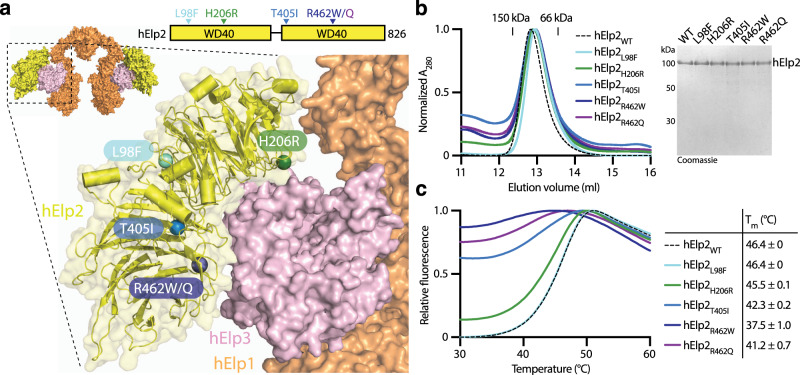
Fig. 2. Mutations found in patients with intellectual disability and autism impair the stability of hElp2 protein.
a Localization of patient-derived mutations in the context of human ELP123 (hElp123) model prepared using Cryo-EM structure of yeast Elp123 (yElp123; PDB 6QK7). Color code for proteins: hElp1—orange, hElp2—yellow, hElp3—pink. Mutations are depicted in different colors on hElp2 domain organization scheme and a close-up of hElp123 model. b Purification of wild-type (WT) and mutant hElp2. Gel filtration profiles from S200 Increase 10/300 GL column (left) and Coomassie-stained SDS-PAGE gel showing purified hElp2 variants (right; n = 3 independent experiments). c Averaged melting curves from thermal shift assay for hElp2 variants with calculated melting temperatures (Tm) (mean ± SD; n = 3 independent measurements). Source data are provided as a Source Data file.