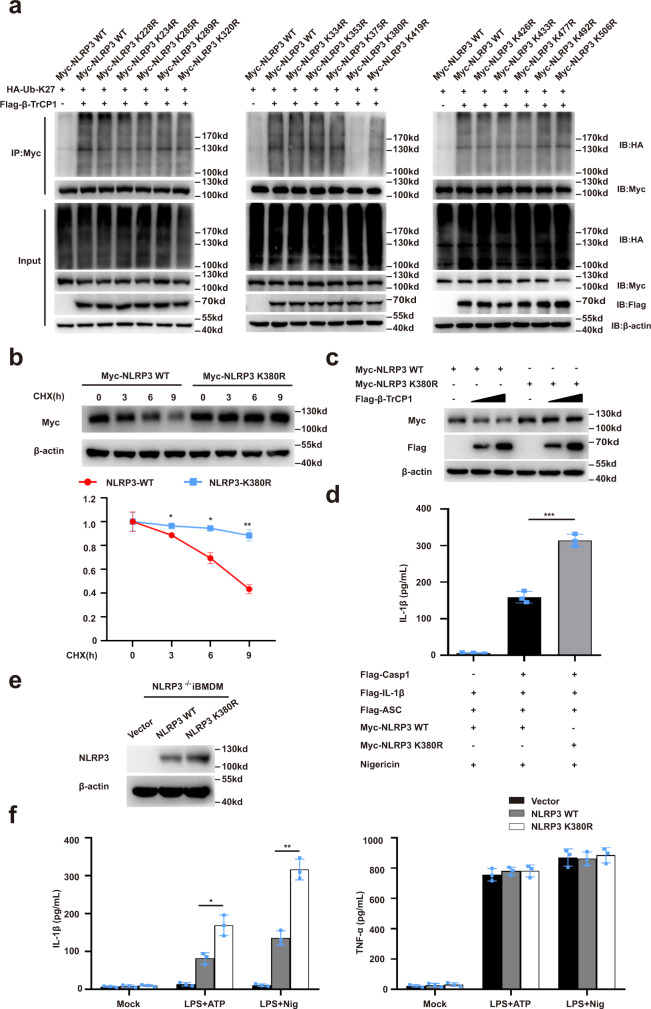
Fig. 7. Lys380 in NLRP3 is essential for its ubiquitination and degradation.
a Immunoblot analysis of lysates from HEK293T cells transfected with HA-tagged K27-linked ubiquitin (K27-Ub), β-TrCP1 and Myc-NLRP3 or indicated mutant Myc-NLRP3, followed by IP with anti-Myc, probed with anti-HA. b Immunoblot analysis of lysates from HEK293T cells transfected with Myc-NLRP3, Myc-NLRP3 K380R, and then treated for various times with cycloheximide (CHX, 100 ug/mL) (top). NLRP3 and NLRP3 K380R expression levels were quantitated by measuring band intensities using “ImageJ” software. The values were normalized to actin (bottom) (mean ± SD, two-way ANOVA with Bonferroni test, NLRP3-K380R vs. NLRP3-WT lower panel: *P = 0.0129, *P = 0.0321, **P = 0.0010 in sequence; n = 3 independent experiments). c Immunoblot analysis of lysates from HEK293T cells transfected with Flag-β-TrCP1 and Myc-NLRP3 or Myc-NLRP3 K380R. d ELISA of IL-1β in supernatants from HEK293T cells transfected with ASC, pro-caspase-1, pro-IL-1β, NLRP3 or NLRP3 K380R plasmids and stimulated with 10 μM nigericin (mean ± SD, one-way ANOVA with Bonferroni test, the third group vs. second group ***P < 0.0001; n = 3 independent experiments). e, f NLRP3−/− iBMDMs were infected with lentivirus expressing mouse WT or NLRP3-K380R, respectively. Immunoblot analysis of indicated proteins (e), then treated these cells with LPS plus ATP or nigericin for detection of IL-1β and TNF-α in supernatants (f) (mean ± SD, two-way ANOVA with Bonferroni test, NLRP3-K380R vs. NLRP3-WT left panel: *P = 0.0475, **P = 0.0037 in sequence, n = 3 independent experiments). Similar results were obtained from three independent experiments. Source data are provided as a Source Data file.