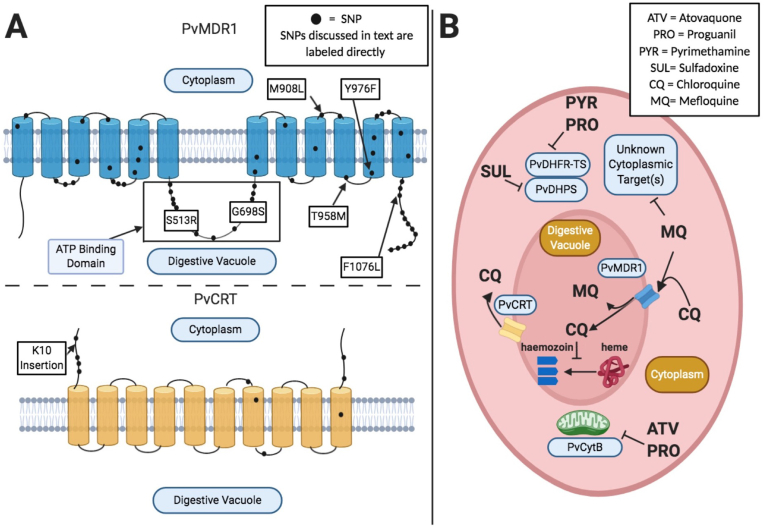
Fig. 2.
A) Depicts the putative structures of PvMDR1 and PvCRT including their predicted transmembrane domains and where observed polymorphisms are positioned within the proteins. Specific SNPs discussed in text are labeled, while other observed mutations are shown as a black dot. Transmembrane domain locations are adapted from Sá et al., 2005 for PvMDR1, and Nomura et al., 2001 for PvCRT. SNP locations are approximate and inferred based on location in protein sequence. B) Depicts putative mechanisms of antimalarial compound action and suspected molecular resistance mechanisms. PvMDR1 and PvCRT are digestive vacuole (DV) transmembrane proteins. Based on orthology to P. falciparum, it is thought that PvMDR1 mutants confer CQ resistance by reducing transport of CQ into the digestive vacuole. PvCRT is thought to act as an efflux pump; actively transporting CQ out of the DV and away from its target (the process of converting heme into hemozoin). PvMDR1 is thought to confer resistance to MQ and possibly other compounds, by transporting them into the DV, thereby sequestering them away from their putative cytoplasmic targets.