Abstract
Background & Aims
Smaller 8-mm diameter transjugular intrahepatic portosystemic shunts (TIPS) appear to be more beneficial than larger 10-mm TIPS stent-grafts, but lack the ability for secondary dilation in cases of clinical ineffectiveness. Underdilated VIATORR® TIPS stent grafts (VTS) expand passively, whereas novel VIATORR Controlled Expansion (VCX) stent grafts do not. This study evaluated the impact on survival of underdilated VCX compared with VTS in patients with decompensated cirrhosis.
Methods
This was a prospective case-control study including patients with cirrhosis receiving TIPS using 10-mm VCX underdilated to 8 mm. Patients with cirrhosis receiving 10-mm VTS underdilated to 8 mm were matched for age, sex, indication for TIPS, and liver function.
Results
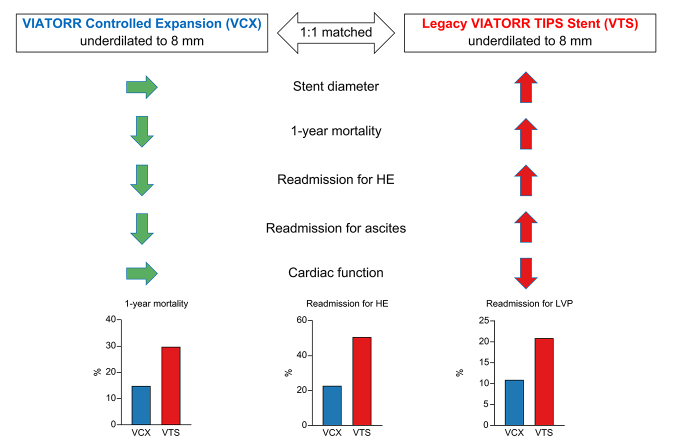
A total of 114 patients (47 VCX, 47 VTS, and 20 fully dilated VCX/VTS) were included. After TIPS implantation, underdilated VCX diameter was 8.0 (7.8–9.2) mm at a median time of 359 (87–450) days, compared with VTS at 9.9 (9.7–10.0) mm (p <0.001). The portosystemic pressure gradient immediately after TIPS procedure and after 7 days did not change significantly in VCX [mean 9.4 (± 0.8) vs. 10.4 (± 0.7) mmHg, p = 0.115). Hospital readmission rates for hepatic encephalopathy were 23% (n = 11) vs 51% (n = 24) for VCX and VTS (p <0.001), respectively. Patients with VCX had significantly lower rates of large-volume paracentesis (n = 5 [11%] vs. n = 10 [21%], p = 0.017) and heart failure (n = 1 [2%] vs. n = 7 [15%], p = 0.015). One-year mortality for underdilated VCX and VTS was 15% (n = 7) and 30% (n = 14) and, for fully dilated VCX/VTS, was 45% (n = 9) (log-rank p = 0.008), respectively.
Conclusions
This study demonstrated that VCX stent grafts underdilated to 8 mm do not passively expand to nominal diameter and suggests reduced hospital readmissions because of hepatic encephalopathy, uncontrolled ascites, and heart failure, and improved 1-year survival compared with underdilated VTS.
Lay summary
Transjugular intrahepatic portosystemic shunt (TIPS) improves survival in selected patients with liver cirrhosis and acute variceal bleeding or refractory ascites. Smaller 8-mm diameter TIPS stent grafts appear to improve patient outcome compared with larger 10-mm diameter stent grafts. Novel VIATORR® Controlled Expansion (VCX) stent grafts facilitate safe and stable underdilation to 8 mm of large 10-mm diameter stent grafts with improved patient outcome (survival, hepatic encephalopathy, ascites and heart failure) compared with legacy VIATORR TIPS stent graft (VTS). Thus, the use of underdilated VCX could preserve heart function.
Clinical Trials Registration
The study is registered at Clinicaltrials.govNCT03628807.
Keywords: Cirrhosis, Liver, Acute decompensation, Transjugular intrahepatic portosystemic shunt, TIPS, Hepatic encephalopathy, Ascites
Abbreviations: CT, computed tomography; HE, hepatic encephalopathy; HF, heart failure; LV, left ventricular; LV-GLS, LV global longitudinal strain; LVP, large-volume paracentesis; MELD, model of end-stage liver disease; NEPTUN, Non-invasive Evaluation Program for TIPS and follow Up Network; PSPG, portosystemic pressure gradient; PTFE, polytetrafluorethylene; RA, recurrent/refractory ascites; RAAS, renin-angiotensin-aldosterone system; SPSS, spontaneous portosystemic shunt; TIPS, transjugular intrahepatic portosystemic shunt; TTE, transthoracic echocardiography; VCX, VIATORR controlled expansion; VTS, VIATORR TIPS stent; VB, variceal bleeding
Graphical abstract
Highlights
-
•
Novel VIATORR® Controlled Expansion (VCX) stent grafts facilitate safe and stable underdilation to 8 mm of large 10-mm diameter stent-grafts.
-
•
Use of underdilated VCX improved outcome (survival, hepatic encephalopathy, ascites, and heart failure) compared with legacy VIATORR TIPS stent grafts (VTS).
-
•
Use of underdilated VCX preserved cardiac function compared with VTS.
Introduction
In the natural history of liver cirrhosis, complications of portal hypertension, such as variceal bleeding (VB), recurrent/refractory ascites (RA) and hepatic encephalopathy (HE), are the main causes of acute decompensation, which lead to high morbidity and mortality.[1], [2], [3], [4], [5], [6] Transjugular intrahepatic portosystemic shunt (TIPS) increases survival in selected patients to the point that it has become an essential therapeutic option in the treatment of acute VB and RA.1,[7], [8], [9], [10], [11], [12], [13], [14] A key factor for the widespread use of TIPS has been the introduction of polytetrafluorethylene (PTFE)-covered stent grafts since the start of the 21st century, which has markedly reduced the rates of shunt dysfunction and the consequent risk of clinical relapse of portal hypertensive complications.15,16 However, the risk of HE after TIPS placement remains a clinically relevant problem.11
The use of smaller 8-mm compared with larger 10-mm nominal diameter stent grafts has been shown to reduce HE with similar effectiveness in the setting of secondary prevention of VB.17 By contrast, an early interrupted randomised study showed that 8-mm nominal diameter stent grafts were associated with a higher recurrence of ascites and with the same rate of HE compared with 10-mm nominal diameter stent grafts.18 Similar conclusions were drawn by a recent retrospective study, suggesting a better control of ascites in patients treated with 10-mm nominal diameter stent grafts.19 Moreover, using smaller nominal diameter stent grafts is limited by further dilatation to a larger diameter in case of haemodynamic and clinical ineffectiveness. A strategy used to overcome this problem is the underdilatation of 10-mm nominal diameter stent grafts to 8 mm.10 However, both the classic covered and uncovered 10-mm nitinol stents are supposed to passively expand to full nominal diameter at least when underdilated to 8 mm.[20], [21], [22], [23] Recently, a national registry study demonstrated a survival benefit of the use of smaller 8-mm compared with larger 10-mm diameter TIPS in matched patients, as well as of 10-mm stent grafts underdilated to 8 mm.24
Indeed, certain windows of portosystemic pressure gradient (PSPG) have been suggested to be beneficial for patient outcomes. Therefore, an excessive reduction in the PSPG (<10 or 8 mmHg) using a large-diameter TIPS, as well as an insufficient PSPG reduction (>12 mmHg) using a small-diameter TIPS might be unfavourable.8,[25], [26], [27]
According to the manufacturer (W.L. Gore & Associates, Phoenix, AZ, USA), the novel VIATORR® Controlled Expansion (VCX) stent graft has a 10-mm nominal diameter, but can be underdilated to 8 or 9 mm. An extra sleeve prevents its passive expansion and the stent graft can be further dilated if needed.28,29 However, a comparison of the clinical efficacy of underdilated VCX compared with the first-generation VIATORR TIPS Stent-graft (VTS) has not previously been performed. Therefore, the current study evaluated the impact of underdilated VCX compared with VTS on the survival of patients with decompensated liver cirrhosis.
Methods
Study population and design
Patients above 18 years of age with decompensated liver cirrhosis who underwent a TIPS procedure using a VCX stent-graft underdilated to 8 mm were prospectively enrolled from May 2016 to August 2017. All cases were retrospectively matched 1:1 for age, sex, aetiology of cirrhosis, indication for TIPS, previous episodes of HE and model for end-stage liver disease (MELD) score with patients from the prospective observational Non-invasive Evaluation Program for TIPS and follow Up Network (NEPTUN) cohort, (ClinicalTrials.gov Identifier: NCT03628807),[30], [31], [32] who underwent TIPS placement using 10-mm nominal diameter VTS underdilated to 8 mm from 2013 to 2016. Moreover, an additional group, whose TIPS was created using either a VCX or VTS stent graft fully dilated to 10 mm, was also identified from the NEPTUN cohort.
Primary outcome was 1-year survival; secondary outcomes were hospitalisations for complications, such as HE (assessed by West-Haven criteria3), RA (with the need for large-volume paracentesis [LVP] defined as >5 L per day33), infections/sepsis34 and heart failure (HF). Clinical signs of HF were defined similarly as previously described by Billey et al.,35 including the development of peripheral oedema and anasarca associated or not with ascites, or pleural effusion needing LVP 2 months after TIPS. The patients were followed for 1 year after having undergone the TIPS procedure.
The local ethics committee of the University of Bonn approved the study (029/13), and all patients agreed and signed an informed written consent in accordance with the Helsinki Declaration for the procedures they underwent. The study is registered at ClinicalTrials.gov (NCT03083925).
Implantation of TIPS and portosystemic pressure gradient measurement
The TIPS procedure was performed as previously described.11,24,[36], [37], [38] All included patients received a 10-mm nominal diameter VIATORR (VCX or VTS depending on the group). The dilation of the stent graft was performed using balloon catheters according to the manufacturer’s instructions for use. For underdilated VCX and VTS, a non-compliant balloon (8 × 40 mm Mustang; Boston Scientific, Galway, Ireland) was used in all cases, with inflation to the nominal pressure of 10 bar (BasixCOMPAK, Merit Medical Systems, Inc, South Jordan, UT, USA). The TIPS procedure and haemodynamic measurements were routinely performed under on-demand analgesia with pethidine and without sedation, mechanical ventilation, or vasoactive drugs.
Before and after TIPS placement, portal and central venous pressures were measured via a transducer system and a multichannel monitor. The primary goal was to reduce the PSPG by at least 50% of pre-TIPS value in patients with RA or to <12 mmHg in patients with VB with an initial stent dilation to 8 mm. If the reduction in target pressure was not achieved, then further dilation to 10 mm was performed. Optimal TIPS function was assumed when adequate reduction of PSPG was achieved and portal perfusion of both the liver and collaterals were not evident at final angiography. A second PSPG measurement was performed 7 days after TIPS placement in a subgroup of patients with a VCX stent graft.
Stent graft diameter measurement
Stent graft diameters from all patients from the underdilated VCX group (and 30 patients with available computed tomography [CT] scans from the underdilated VTS group) were measured by CT scans performed after the TIPS procedure either for hepatocellular cancer screening or for clinical events. CT scans were reconstructed and TIPS diameter was measured at 10 sections along the entire PTFE-covered part of the graft, including portal and hepatic venous entrance, and the mean diameter was calculated.20 Stent-graft diameter at baseline was assessed using a dilation balloon.
Cardiac function
For evaluation of cardiac function, transthoracic echocardiography (TTE) was performed before and 6 weeks after TIPS placement in a subgroup of matching patients who received underdilated VCX or VTS TIPS, respectively. Commercially available equipment (Vivid 7 [General Electric Medical Health, Waukesha, WI, USA] and iE33 [Philips Medical Systems, Koninklijke N.V., Heerlen, The Netherlands]) with a 2.5-MHz phased-array transducer were used as previously described.39 Speckle-tracking echocardiography was additionally performed to evaluate left ventricular (LV) myocardial contractility. The endocardial boundary in the left ventricle was set manually. The LV global longitudinal strain (LV-GLS) was calculated and normal values (-21% ± 1) set as previously described.39,40 Image Arena 4.3 (TomTec Imaging Systems GmbH, 2001-2010, Unterschleissheim, Germany) was used to automatically measure frame-by-frame myocardial shortening of a distance between 2 points on the myocardium. Diastolic dysfunction was ruled out via TTE in all patients before receiving TIPS.
Statistical analysis
Descriptive statistics were performed for all variables. Non-parametric testing was used to compare groups when suitable. Paired non-parametric testing was used to compare data before and after TIPS procedure in the same patients. Survival analysis was performed using Kaplan-Meier survival curves with log-rank tests. Analysis of decompensation (RA or HE) was performed using the Fine and Gray method, with death and liver transplantation as a competing risk.41 Univariate and multivariate regression analyses were performed to identify risk factors. The multivariate analysis included all values with p <0.05. Continuous variables are presented as median (range). Categorical variables are presented as absolute cases or percentages. All data were analysed using SPSS (version 24, IBM, Armonk, NY, USA).
All authors had access to the study data and reviewed and approved the final manuscript.
Results
General characteristics of the patients
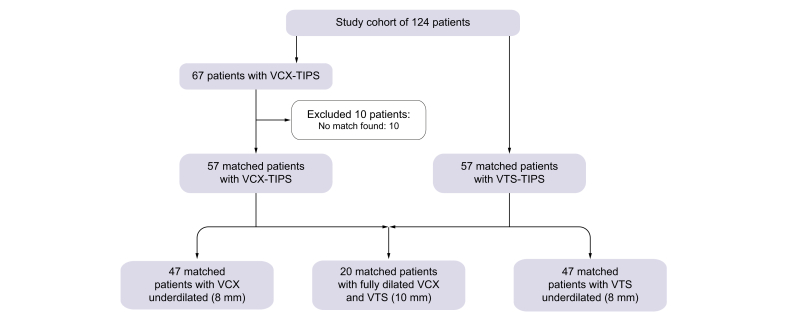
In total, 47 consecutive patients with decompensated liver cirrhosis undergoing TIPS placement with 10-mm VCX underdilated to 8 mm were compared with 47 suitable matches receiving 10-mm VTS underdilated to 8 mm. A further 20 patients (10 for each type of stent graft) with TIPS fully dilated to 10 mm were included as a reference group in the survival analysis (Fig. 1).
Fig. 1.
Flow chart of patient cohorts included in the study.
VCX, VIATORR controlled expansion; VTS, first generation VIATORR stent-graft.
Median age at TIPS procedure was 58 (28–81) years (underdilated VCX group 59 [29–81] years). The most common aetiologies of cirrhosis were alcohol (n = 68/94, 72%) and chronic HBV and/or HCV (n = 8/94, 9%). RA was the indication for TIPS in 72/94 patients (77%), whereas 22/94 (23%) received pre-emptive TIPS for secondary prophylaxis of VB.14 Median MELD was 10 (6–23) and median Child-Pugh score was 8 (5–13). Median follow-up time was 365 (7–365) days. There was no significant difference in general characteristics between the groups (Table 1).
Table 1.
General characteristics of the study population.
| Parameter | VCX underdilated (n = 47) | VTS underdilated (n = 47) | VCX or VTS fully dilated (n = 20) |
|---|---|---|---|
| General at baseline | |||
| Age (in years) | 59 (29–81) | 58 (23–75) | 55 (37–68)∗∗ |
| Sex (m/f) | 26/21 (55/45%) | 26/21 (53/47%) | 9/11 (45%) |
| Aetiology of cirrhosis (alcoholic/viral/others) | 34/4/9 (72/9/19%) | 34/4/9 (72/9/19%) | 15/3/2 (75/15/10%) |
| TIPS indication (bleeding/ascites) | 11/36 (23/77%) | 11/36/0 (23/77%) | 4/16 (20/80%) |
| Clinical history | |||
| Hepatic encephalopathy | 2 (4%) | 2 (4%) | 1 (5%) |
| Ascites | 40 (85%) | 41 (87%) | 19 (95%) |
| Variceal bleeding | 17 (36%) | 18 (38%) | 6 (30%) |
| Scores at baseline | |||
| MELD | 11 (6–25) | 10 (6–23) | 10 (7–25) |
| Child-Pugh | 8 (5–13) | 8 (5-12) | 8 (5–11) |
| Child-Pugh class A/B/C | 6/27/14 (13/57/30%) | 3/34/10 (6/72/21%) | 2/14/4 (10/70/20%) |
| Laboratory results at baseline | |||
| Sodium [mmol/L] | 137 (125–146) | 137 (117–146) | 138 (129–148) |
| Creatinine [mg/dl] | 1.5 (0.6–6.7) | 1.2 (0.5–4.2) | 1 (0.7–6.1) |
| Bilirubin [mg/dl] | 2 (0.2–11) | 1.5 (0.4–6.8) | 1.1 (0.3–6.8) |
| Aspartate aminotransferase [U/L] | 56 (8–131) | 43 (16–115)∗∗ | 24 (14–46) |
| Alanine transaminase [U/L] | 36 (8–131) | 29 (8–99) | 36 (20–95) |
| C-reactive protein (mg/L) | 22 (0.8–153) | 19 (0.2–120) | 7.6 (0.2–77) |
| Albumin (g/L) | 29 (3–48) | 31 (4–46) | 33 (8–48) |
| International normalised ratio | 1.2 (0.9–1.9) | 1.2 (0.9–2.4) | 1.2 (0.9–2.3) |
| White blood cell count [10³/μl] | 8 (2–47) | 6 (1–14)∗ | 6.5 (1.2–119) |
| Platelets [×109/L] | 143 (16–377) | 151 (25–527) | 125 (28–682) |
| Haemodynamics | |||
| PSPG before TIPS | 19 (12–34) | 19 (12–34) | 18 (12–38) |
| PSPG after TIPS | 9 (4–16) | 9 (2–24) | 10 (4–15) |
| Outcome | |||
| 1-year mortality | 7 (15%) | 14 (30%)∗∗ | 9 (45%)∗ |
| 1-year readmission for heart failure | 1 (2%) | 7 (15%) | 3 (15%)∗ |
MELD, model of end-stage liver disease; PSPG, portosystemic pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt; VCX, VIATORR controlled expansion; VTS, VIATORR TIPS stent. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001 (non-parametric).
Survival after TIPS
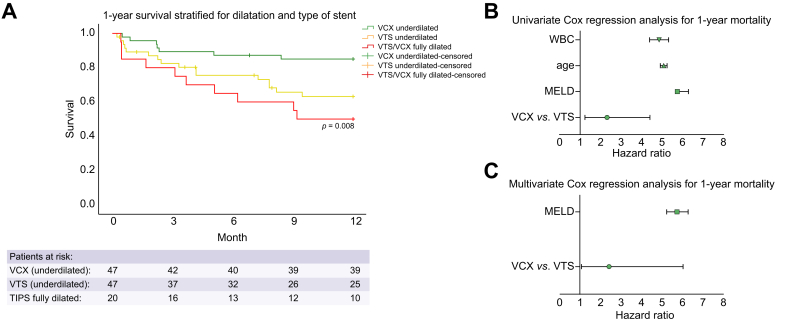
In the 8-mm underdilated VCX and VTS groups, 7 (15%) and 14 (30%) patients died within 1-year follow-up, respectively. In the 10-mm fully dilated group, 9 (45%) patients died during the same follow-up time. One-year survival was significantly higher in patients who received underdilated VCX and was intermediate in those with underdilated VTS. Indeed, the lowest survival was recorded in patients with stent grafts fully dilated to 10 mm (Fig. 2A; competing risk analysis in Fig. S1). Most common causes of death were acute-on-chronic liver failure (n = 16/30, 50%) and sepsis (n = 13/30, 47%) (Table S1). Only 3 patients underwent liver transplantation, and 4 patients were lost to follow-up.
Fig. 2.
One-year survival analysis.
(A) Kaplan-Meier survival curve for 1-year survival stratified by stent type. VCX underdilated to 8 mm (green), VTS underdilated to 8 mm (yellow), VCX and VTS stent grafts fully dilated to 10 mm (red). p by log-rank. Crosses are censor events. X-axis shows time between TIPS and death in months. Y-axis shows 1-cumulative incidence. (B) Univariate Cox regression analysis for 1-year survival. Plot shows HR and 95% CI. (C) Multivariate Cox regression analysis for 1-year survival. Plot shows HR and 95% CI. HR, hazard ratio; MELD, model of end-stage liver disease; VCX, VIATORR controlled expansion, underdilated 8 mm; VTS, first-generation VIATORR stent-graft, underdilated 8 mm; WBC, white blood cell count.
For 1-year mortality after TIPS, use of underdilated VCX over underdilated VTS and pre-TIPS MELD were significant on univariable analysis. Multivariable analysis confirmed the use of underdilated VCX and MELD as independent predictors of 1-year survival (Fig. 2B,C and Table 2).
Table 2.
Cox regression analysis for 1-year mortality.
| Parameter | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| VTS vs. VCX underdilated | 0.011 | 2.316 | 1.210–4.432 | 0.047 | 2.420 | 1.073–6.016 |
| MELD | 0.002 | 1.150 | 1.150–1.257 | 0.003 | 1.145 | 1.047–1.252 |
| Age | 0.281 | 1.017 | 0.986–1.049 | |||
| WBC | 0.560 | 0.972 | 0.885–1.068 | |||
HR, hazard ratio; MELD, model of end-stage liver disease; VCX, VIATORR controlled expansion; VTS, VIATORR TIPS stent; WBC, white blood cell count.
Stent graft expansion and portosystemic pressure gradient before and after VCX stent graft placement
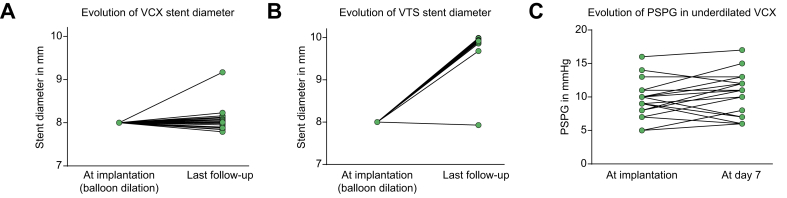
The CT scan reconstructions performed on the 47 underdilated VCX stent grafts showed a lack of significant passive expansion in most patients, with a median diameter of 8.0 (7.8–9.2) mm at a median time of 359 (87–450) days, well after the time frame of 6 weeks for the passive expansion of VTS stents, as previously shown (Fig. 3A).20 At the sites of portal and hepatic venous entrance, the median diameters were similar, at 8.0 (7.4–8.4) mm and 8.0 (7.5–8.5) mm, respectively. Except for 1 case, the median diameter of PTFE-covered sections in underdilated VTS increased to 9.9 (9.7–10.0) mm at a median time of 66 (1–1547) days (Fig. 3B). Portal and hepatic venous entrance sites showed median diameters of 10.0 (9.7–10.0) mm and 10.0 (9.5–10.0) mm, respectively.
Fig. 3.
Evolution of median stent diameter measured by computed tomography reconstruction at last follow-up.
X-axes show time of measurement. (A) VCX (n = 47). Y-axis shows diameter in mm. (B) VTS (n = 30). Y-axis shows diameter in mm. (C) Evolution of PSPG of patients with a VCX immediately after TIPS implantation and 7 days post implantation (n = 21). Y-axis shows PSPG in mmHg. PSPG, portosystemic pressure gradient; VCX, VIATORR controlled expansion; VTS, first-generation VIATORR stent graft.
A second PSPG measurement 7 days after TIPS placement was performed in 21 patients receiving VCX, and showed a non-significant increase compared with immediate post-TIPS values (mean 9.4 [± 0.8] vs. 10.4 [± 0.7] mmHg, p = 0.115) (Fig. 3C).
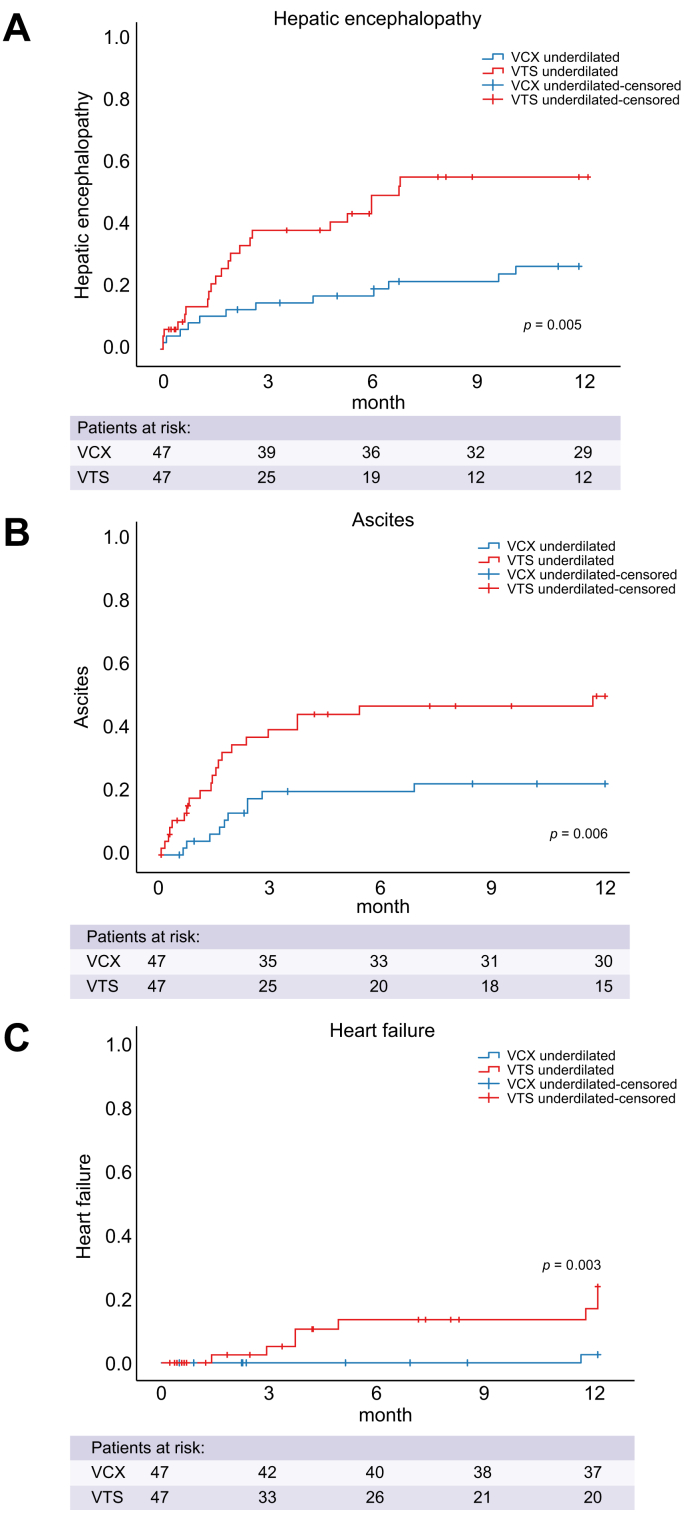
Main decompensations after TIPS
During follow-up, 35 patients (37%) developed, and were hospitalised for, at least 1 episode of HE, with significantly lower rates in the underdilated VCX group (underdilated VCX n = 11 [23%] vs. underdilated VTS n = 24 (51%), p <0.001). Of those patients, 13 had HE of West-Have-Grade II or higher (Fig. 4A).
Fig. 4.
Time-to-event analysis for hospitalisation for (A) hepatic encephalopathy, (B) large-volume paracentesis and (C) heart failure.
X-axes show time between TIPS and hospitalisation in months. Y-axes show cumulative incidence. p by log-rank. Blue indicates patients with a VCX implanted; red indicates patients with a VTS implanted. TIPS, transjugular intrahepatic portosystemic shunt; VCX, VIATORR controlled expansion; VTS, first-generation VIATORR stent graft.
Similarly, 15 (16%) of patients required recurrent LVP (0.2 LVP per patient). Patients with VCX had significantly lower rates of paracentesis during follow-up [n = 5 (11%), 0.1 LVP per patient vs. n = 10 (21%), 0.3 LVP per patient; p = 0.017] (Fig. 4B). Of those patients, 2 patients (20%) in the VTS group and none (0%) in the VCX group were also admitted for HF.
Hospitalisation for HF was recorded in 11 (5%) patients. The underdilated VCX group showed a low rate of HF (n = 1, 2%) compared with patients from the underdilated VTS and the fully dilated stent graft groups (n = 7, 15% and n = 3, 15%, p = 0.027, respectively) (Table 1 and Fig. 4C).
During 12 months of follow-up, VB developed in only 1 patient in the underdilated VTS group and none in the other 2 groups. Competing risk analyses for hospitalisation for HE, LVP, and HF with death and liver transplantation as competing events confirmed these data (Fig. S2A–C).
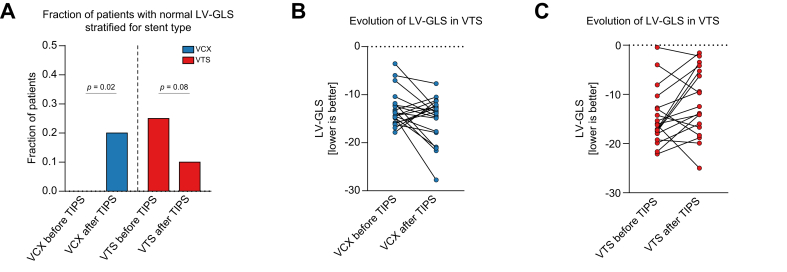
Cardiac function
LV function was assessed via speckle-tracking echocardiography in 20 matched patients from the underdilated VCX and VTS groups. Before TIPS, there was no significant difference in LV-GLS between the 2 groups (Fig. 5B,C). However, only 5 (12.5%) patients presented a LV-GLS within normal ranges. All 5 patients were in the VTS group (Fig. 5A) After TIPS, LV-GLS improved to normal range in 4 (20%) patients in the VCX group, which was statistically significant. In the VTS group, only 2 (10%) patients remained within normal LV-GLS ranges (Fig. 5A). Analysis of the absolute values of LV-GLS showed that median evolution of LV-GLS improved in the VCX group, but worsened in the VTS group (Fig. 5B,C)
Fig. 5.
Evolution of cardiac function.
(A) Fraction of patients with normal LV-GLS by speckle-tracking echocardiography before and after TIPS. Blue indicates patients with a VCX implanted; red indicates patients with a VTS implanted. Y-axis shows the fraction of patients. p by paired non-parametric testing. Evolution of LV-GLS before and 6 weeks after TIPS implantation in patients receiving (B) VCX or (C) VTS. Y-axes show LV-GLS in percent. LV-GLS, left ventricular global longitudinal strain; TIPS, transjugular intrahepatic portosystemic shunt; VCX, VIATORR controlled expansion; VTS, first-generation VIATORR stent graft.
Discussion
This study suggests that creation of TIPS using a 10-mm VCX stent-graft underdilated to 8 mm improved 1-year survival compared with a 10-mm VTS underdilated to 8 mm and with fully dilated stent grafts. Moreover, the use of underdilated VCX stent grafts reduced the rate of shunt-related complications, including HE, need for delayed LVP and HF. We consider this to be a likely consequence of the maintenance of a small TIPS calibre, which guarantees the stability of the shunted blood volume from the splanchnic bed to the heart and the systemic circulation.
The proper use of TIPS has been a matter of debate in clinical hepatology for the past few decades. Not only indications and timing for TIPS, but also technical issues regarding both the target pressure gradient to be reached and the right way to measure it26,[42], [43], [44] as well as the best calibre of the shunt, have been addressed.[17], [18], [19],24
Although the relationship between portosystemic shunting and HE is well established, no clear-cut data exist for the relationship between TIPS calibre and survival. Indeed, recent studies showed a clear correlation of larger calibre stent grafts with higher rates of HE, favouring smaller diameter stent grafts.12,13,45 Interestingly, just as larger calibre and uncontrolled expansion TIPS in the current study affected the risk for HE and survival, the total area of a spontaneous portosystemic shunt (SPSS) of >83 mm2, which translates to a shunt diameter of 10.3 mm, is likewise independently associated with a higher risk for HE and death in liver cirrhosis.46 Although speculative, the current data and those on SPSS support the hypothesis that the safety windows of shunt calibre quickly close for diameters >8 mm.
The use of 8-mm nominal-diameter stent grafts is limited by secondary dilatation to a larger diameter in case of haemodynamic and clinical ineffectiveness. The current study confirmed VCX stent grafts as a viable solution to obtain a stable 8-mm shunt that can be further dilated if needed.28 Of note, a study using VTS (either 8- or 10-mm nominal diameter) that were underdilated to 6 or 7 mm, although observing passive expansion in several patients, showed beneficial effects compared with fully dilated stent grafts.23 These data might be reconciled by the presented study, which showed an intermediate effect on survival of underdilated VTS compared with underdilated VCX and fully dilated stent grafts. Interestingly, a difference in survival was already noticeable within the first 6 weeks, parallel to the timing of the passive expansion of VTS stent grafts, as shown previously.20
Cardiac function and haemodynamics, especially hyperdynamic circulation, have been identified as crucial determiners of the outcome of patients with decompensated liver cirrhosis.39,40,[47], [48], [49] Hyperdynamic circulation has been linked not only with systemic inflammation,47,48 but also with TIPS implantation.11,50,51 TIPS dramatically redirects portal venous blood flow to the right atrium, which increases cardiac preload and might precipitate heart decompensation after TIPS.50,51 Myocardial impairment is common in patients with liver cirrhosis, especially in decompensated stages.49 This is well reflected in the current results, which showed abnormal LV-GLS in most of the study patients. A recent study reported high rates (20%) of cardiac decompensation and hospitalisation of patients receiving fully dilated 10-mm VTS.35 The use of stent grafts with a smaller diameter would also provide a benefit in terms of reducing cardiac load and preserving cardiac function. The current data suggested a preserved cardiac function by using a smaller 8-mm VCX TIPS stent (which stays at an 8-mm diameter) compared with the legacy VTS stent, which passively expands to a 10-mm diameter. In HF, a pathophysiology of decreased effective arterial blood volume because of decreased cardiac output results in the stimulation of the renin-angiotensin-aldosterone system (RAAS) and finally sodium retention, manifesting in the development of ascites. In decompensated cirrhosis, RAAS is already activated, and sodium is retained. Thus, cardiac decompensation after TIPS might contribute to TIPS-resistant ascites despite the reduction in PSPG.
The current results from speckle tracking echocardiography39,40,52 suggested an improvement in cardiac contractility 6 weeks after TIPS placement only in patients receiving underdilated VCX. However, the number of cardiac events was lower in the current study, limiting the drawing of final conclusions. Despite suggesting an impact on cardiac function, a complete cardiac evaluation, including right heart catheterisation, was not performed, therefore limiting the generalisation of the current results.
Myocardial impairment has been linked to sarcopaenia.53 Although sarcopaenia has been reported as a risk factor for the development of HE and mortality,30,31,54,55 TIPS has been shown to improve sarcopaenia in some patients with TIPS.31,56 Determining the effect of smaller TIPS diameter on sarcopaenia was beyond the scope of the current study and should be explored in future research. Interestingly, portosystemic shunting has been linked with the development of sarcopaenia, suggesting beneficial effects of a smaller shunt diameter.57 Patient age is a known risk factor for sarcopaenia and outcome in general. Importantly, the use of underdilated VCX is associated with higher survival compared with underdilated VTS independently of age, further suggesting a significant impact of smaller TIPS diameter on survival.
Previous episodes of HE are also a known risk factor for the development of HE after TIPS.58 In the current study, both groups (VCX and VTS) were matched for this risk factor. This would suggest that the result of excess rates of HE development after TIPS was associated with the use of a smaller TIPS diameter (underdilated VCX), which is in line with previous reports and the clinical practice of reduction or occlusion of TIPS/SPSS in patients with refractory HE.[59], [60], [61], [62]
One of the strongest predictors of outcome of decompensated cirrhosis with and without TIPS is the PSPG . The current study demonstrated the lack of a decrease in the PSPG 7 days after TIPS in patients receiving the VCX stent graft. The crucial role of a stable and personalised PSPG is underlined by studies demonstrating that a narrow beneficial window for PSPG after TIPS is thought to be associated with the best outcome.11,[25], [26], [27] It is expected that, with the use of VCX, clinicians can better modulate the PSPG at TIPS placement and during follow-up. Thus, the current study supports and expands all the existing data of the benefit of the use of controlled expansion stent grafts to create a TIPS.
Despite a well-characterised prospective cohort, the current study has several limitations. It presents a monocentric study without randomisation, and control matching was performed retrospectively, although from the prospective observational NEPTUN cohort. Thereby, not all confounding variables and potential selection factors to either 8 or 10-mm dilatation might have been detected. In our cohort, most patients (80%) had >4 LVPs before allocation to TIPS, suggesting that they were more advanced in their liver disease progression compared with other studies. Thus, the mortality in our cohort was higher than in recent RCTs, although similar to recent observational cohorts.35,63 However, the results of this study might encourage larger prospective multicentre studies.
Conclusion
This study suggests that the use of underdilated VCX stent grafts improves survival in patients with decompensated cirrhosis and reduces the risk of post-procedural complications compared with first-generation VIATORR stent-grafts.
Financial support
This study was supported by W.L. Gore & Associates Medical. None of the funders had an influence on the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors’ contributions
M.P.: acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis. J.A.O., J.C., C.J., and P.K.: acquisition of data, analysis and interpretation of data. C.P.S. and D.T.: administrative support; F.S., M.M., J.G., C.M., and C.P.: interpretation of data, critical revision of the manuscript regarding important intellectual content, study supervision; J.T.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript regarding important intellectual content, funding recipient, administrative, technical and material support, study supervision.
Data availability statement
Data are available upon request from the authors.
Conflict of interest
M.P. received funding from the Ernst und Berta Grimmke Foundation (Germany) (Lfd.Nr.5/19), BONFOR research program of the University of Bonn, Germany (2020-2A-07). J.T. received funding from the European Union’s Horizon 2020 research and innovation program’s GALAXY study (No. 668031), LIVERHOPE (No. 731875), MICROB-PREDICT (No. 825694), the Cellex Foundation, and W.L. Gore & Associates Medical.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100264.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Angeli P., Bernardi M., Villanueva C., Francoz C., Mookerjee R.P., Trebicka J. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R., Faculty Baveno V.I. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 3.American Association for the Study of Liver Diseases European association for the study of the liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Gustot T., Fernandez J., Garcia E., Morando F., Caraceni P., Alessandria C. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 5.Jalan R., Pavesi M., Saliba F., Amorós A., Fernandez J., Holland-Fischer P. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Ripoll C., Groszmann R., Garcia-Tsao G., Grace N., Burroughs A., Planas R. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Trebicka J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J Hepatol. 2017;66:442–450. doi: 10.1016/j.jhep.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Trebicka J. Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients? Semin Liver Dis. 2018;38:87–96. doi: 10.1055/s-0038-1627457. [DOI] [PubMed] [Google Scholar]
- 9.Bureau C., Thabut D., Oberti F., Dharancy S., Carbonell N., Bouvier A. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 10.García-Pagán J.C., Caca K., Bureau C., Laleman W., Appenrodt B., Luca A. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 11.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Salerno F., Cammà C., Enea M., Rössle M., Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Lv Y., Yang Z., Liu L., Li K., He C., Wang Z. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Gea V., Procopet B., Giráldez Á., Amitrano L., Villanueva C., Thabut D. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology. 2019;69:282–293. doi: 10.1002/hep.30182. [DOI] [PubMed] [Google Scholar]
- 15.Bureau C., Pagan J.C.G., Layrargues G.P., Metivier S., Bellot P., Perreault P. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.Eesa M., Clark T. Transjugular intrahepatic portosystemic shunt: state of the art. Semin Roentgenol. 2011;46:125–132. doi: 10.1053/j.ro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Lv Y., Bai M., Wang Z., Liu H., He C. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67:508–516. doi: 10.1016/j.jhep.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Riggio O., Ridola L., Angeloni S., Cerini F., Pasquale C., Attili A.F. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267–272. doi: 10.1016/j.jhep.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Miraglia R., Maruzzelli L., Tuzzolino F., Petridis I., D’Amico M., Luca A. Transjugular intrahepatic portosystemic shunts in patients with cirrhosis with refractory ascites: comparison of clinical outcomes by using 8- and 10-mm PTFE-covered stents. Radiology. 2017;284:281–288. doi: 10.1148/radiol.2017161644. [DOI] [PubMed] [Google Scholar]
- 20.Pieper C.C., Jansen C., Meyer C., Nadal J., Lehmann J., Schild H.H. Prospective evaluation of passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stent grafts – a three-dimensional sonography study. J Vasc Interv Radiol. 2016;28:117–125. doi: 10.1016/j.jvir.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Mollaiyan A., Bettinger D., Rössle M. The underdilation of nitinol stents at TIPS implantation: solution or illusion? Eur J Radiol. 2017;89:123–128. doi: 10.1016/j.ejrad.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Gaba R.C., Parvinian A., Minocha J., Casadaban L.C., Knuttinen M.G., Ray C.E. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol. 2015;26:382–387. doi: 10.1016/j.jvir.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Schepis F., Vizzutti F., Garcia-Tsao G., Marzocchi G., Rega L., De Maria N. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1153–1162. doi: 10.1016/j.cgh.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Trebicka J., Bastgen D., Byrtus J., Praktiknjo M., Terstiegen S., Meyer C. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol. 2019;17:2793–2799. doi: 10.1016/j.cgh.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Harrod-Kim P., Saad W.E., Waldman D. Predictors of early mortality after transjugular intrahepatic portosystemic shunt creation for the treatment of refractory ascites. J Vasc Interv Radiol. 2006;17:1605–1610. doi: 10.1097/01.RVI.0000240651.38289.4B. [DOI] [PubMed] [Google Scholar]
- 26.Silva-Junior G., Turon F., Baiges A., Cerda E., García-Criado Á., Blasi A. Timing affects measurement of portal pressure gradient after placement of transjugular intrahepatic portosystemic shunts in patients with portal hypertension. Gastroenterology. 2017;152:1358–1365. doi: 10.1053/j.gastro.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Casado M., Bosch J., García-Pagán J.C., Bru C., Bañares R., Bandi J.C. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 28.Miraglia R., Maruzzelli L., Di Piazza A., Mamone G., Caruso S., Gentile G. Transjugular intrahepatic portosystemic shunt using the new gore VIATORR controlled expansion endoprosthesis: prospective, single-center, preliminary experience. Cardiovasc Intervent Radiol. 2019;42:78–86. doi: 10.1007/s00270-018-2040-y. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasa R.N., Srinivasa R.N., Chick J.F.B., Hage A., Saad W.A. Transjugular intrahepatic portosystemic shunt reduction using the gore VIATORR controlled expansion endoprosthesis: hemodynamics of reducing an established 10-mm TIPS to 8-mm in diameter. Cardiovasc Intervent Radiol. 2018;41:518–521. doi: 10.1007/s00270-017-1807-x. [DOI] [PubMed] [Google Scholar]
- 30.Praktiknjo M., Clees C., Pigliacelli A., Fischer S., Jansen C., Lehmann J. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Praktiknjo M., Book M., Luetkens J., Pohlmann A., Meyer C., Thomas D. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67:1014–1026. doi: 10.1002/hep.29602. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann J., Praktiknjo M., Nielsen M.J., Schierwagen R., Meyer C., Thomas D. Collagen type IV remodelling gender-specifically predicts mortality in decompensated cirrhosis. Liver Int. 2019;39:885–893. doi: 10.1111/liv.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore K.P., Wong F., Gines P., Bernardi M., Ochs A., Salerno F. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 34.Piano S., Singh V., Caraceni P., Maiwall R., Alessandria C., Fernandez J. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156:1368–1380. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Billey C., Billet S., Robic M.A., Cognet T., Guillaume M., Vinel J.P. A prospective study identifying predictive factors of cardiac decompensation after TIPS: the Toulouse algorithm. Hepatology. 2019;70:1928–1941. doi: 10.1002/hep.30934. [DOI] [PubMed] [Google Scholar]
- 36.Trebicka J., Krag A., Gansweid S., Appenrodt B., Schiedermaier P., Sauerbruch T. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23:1218–1225. doi: 10.1097/MEG.0b013e32834a75dc. [DOI] [PubMed] [Google Scholar]
- 37.Trebicka J., Krag A., Gansweid S., Schiedermaier P., Strunk H.M., Fimmers R. Soluble TNF-alpha-receptors I are prognostic markers in TIPS-treated patients with cirrhosis and portal hypertension. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Praktiknjo M., Lehmann J., Nielsen M.J., Schierwagen R., Uschner F.E., Meyer C. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2:211–222. doi: 10.1002/hep4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jansen C., Schröder A., Schueler R., Lehmann J., Praktiknjo M., Uschner F.E. Left ventricular longitudinal contractility predicts acute-on-chronic liver failure development and mortality after transjugular intrahepatic portosystemic shunt. Hepatol Commun. 2019;3:340–347. doi: 10.1002/hep4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen C., Cox A., Schueler R., Schneider M., Lehmann J., Praktiknjo M. Increased myocardial contractility identifies patients with decompensated cirrhosis requiring liver transplantation. Liver Transpl. 2018;24:15–25. doi: 10.1002/lt.24846. [DOI] [PubMed] [Google Scholar]
- 41.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 42.Rössle M., Blanke P., Fritz B., Schultheiss M., Bettinger D. Free hepatic vein pressure is not useful to calculate the portal pressure gradient in cirrhosis: a morphologic and hemodynamic study. J Vasc Interv Radiol. 2016;27:1130–1137. doi: 10.1016/j.jvir.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 43.La Mura V., Abraldes J.G., Berzigotti A., Erice E., Flores-Arroyo A., García-Pagán J.C. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: a clinical-hemodynamic correlation study. Hepatology. 2010;51:2108–2116. doi: 10.1002/hep.23612. [DOI] [PubMed] [Google Scholar]
- 44.Bosch J., García-Pagán J.C. Calculating hepatic venous pressure gradient: feel free to stay free. J Vasc Interv Radiol. 2016;27:1138–1139. doi: 10.1016/j.jvir.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 45.Sauerbruch T., Mengel M., Dollinger M., Zipprich A., Rössle M., Panther E. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149:660–668. doi: 10.1053/j.gastro.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Praktiknjo M., Simón-Talero M., Römer J., Roccarina D., Martínez J., Lampichler K. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J Hepatol. 2020;72:1140–1150. doi: 10.1016/j.jhep.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Praktiknjo M., Monteiro S., Grandt J., Kimer N., Madsen J.L., Werge M.P. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int. 2020;40:1457–1466. doi: 10.1111/liv.14433. [DOI] [PubMed] [Google Scholar]
- 48.Turco L., Garcia-Tsao G., Magnani I., Bianchini M., Costetti M., Caporali C. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol. 2018;68:949–958. doi: 10.1016/j.jhep.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Isaak A., Praktiknjo M., Jansen C., Faron A., Sprinkart A.M., Pieper C.C. Myocardial fibrosis and inflammation in liver cirrhosis: MRI study of the liver-heart axis. Radiology. 2020;297:51–61. doi: 10.1148/radiol.2020201057. [DOI] [PubMed] [Google Scholar]
- 50.Busk T.M., Bendtsen F., Møller S. Cardiac and renal effects of a transjugular intrahepatic portosystemic shunt in cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:523–530. doi: 10.1097/MEG.0b013e32835d09fe. [DOI] [PubMed] [Google Scholar]
- 51.Busk T.M., Bendtsen F., Poulsen J.H., Clemmesen J.O., Larsen F.S., Goetze J.P. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;314:G275–G286. doi: 10.1152/ajpgi.00094.2017. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong M.J., Gohar F., Dhaliwal A., Nightingale P., Baker G., Greaves D. Diastolic dysfunction on echocardiography does not predict survival after transjugular intrahepatic portosystemic stent-shunt in patients with cirrhosis. Aliment Pharmacol Ther. 2019;49:797–806. doi: 10.1111/apt.15164. [DOI] [PubMed] [Google Scholar]
- 53.Bhanji R.A., Montano-Loza A.J., Watt K.D. Sarcopenia in cirrhosis: looking beyond the skeletal muscle loss to see the systemic disease. Hepatology. 2019;70:2193–2203. doi: 10.1002/hep.30686. [DOI] [PubMed] [Google Scholar]
- 54.Merli M., Giusto M., Lucidi C., Giannelli V., Pentassuglio I., Di Gregorio V. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 55.Nardelli S., Lattanzi B., Torrisi S., Greco F., Farcomeni A., Gioia S. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2016;15:934–936. doi: 10.1016/j.cgh.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 56.Tsien C., Shah S.N., McCullough A.J., Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 57.Dasarathy S., McCullough A.J., Muc S., Schneyer A., Bennett C.D., Dodig M. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai M., Qi X., Yang Z., Yin Z., Nie Y., Yuan S. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26:943–951. doi: 10.1111/j.1440-1746.2011.06663.x. [DOI] [PubMed] [Google Scholar]
- 59.Blue R.C., Lo G.C., Kim E., Patel R.S., Scott Nowakowski F., Lookstein R.A. Transjugular intrahepatic portosystemic shunt flow reduction with adjustable polytetrafluoroethylene-covered balloon-expandable stents using the “sheath control” technique. Cardiovasc Intervent Radiol. 2016;39:935–939. doi: 10.1007/s00270-015-1249-2. [DOI] [PubMed] [Google Scholar]
- 60.Laleman W., Simon-Talero M., Maleux G., Perez M., Ameloot K., Soriano G. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448–2457. doi: 10.1002/hep.26314. [DOI] [PubMed] [Google Scholar]
- 61.He C., Lv Y., Wang Z., Guo W., Tie J., Li K. Association between non-variceal spontaneous portosystemic shunt and outcomes after TIPS in cirrhosis. Dig Liver Dis. 2018;50:1315–1323. doi: 10.1016/j.dld.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Schindler P., Seifert L., Masthoff M., Riegel A., Köhler M., Wilms C. TIPS modification in the management of shunt-induced hepatic encephalopathy: analysis of predictive factors and outcome with shunt modification. J Clin Med. 2020;9:567. doi: 10.3390/jcm9020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piecha F., Radunski U.K., Ozga A.-K., Steins D., Drolz A., Horvatits T. Ascites control by TIPS is more successful in patients with a lower paracentesis frequency and is associated with improved survival. JHEP Rep. 2019;1:90–98. doi: 10.1016/j.jhepr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the authors.