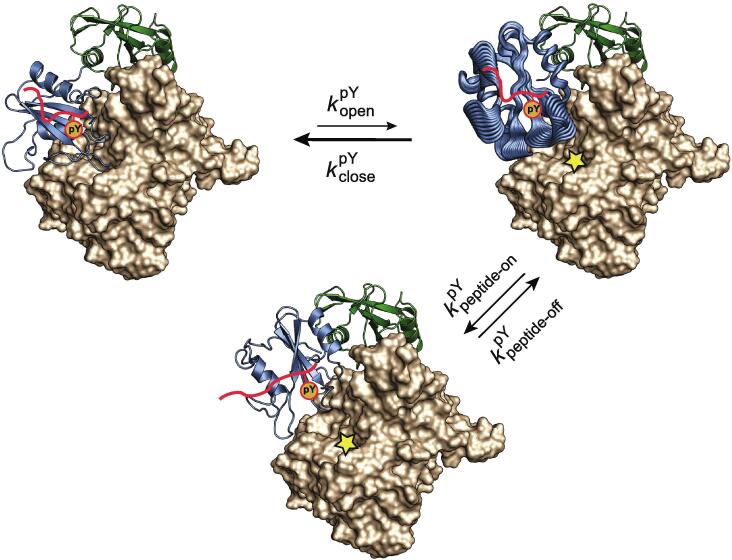
Fig. 9.
A model for the activation of SHP2 by mono-phosphorylated peptides. The N-SH2 domain of SHP2 occludes the catalytic site of the PTP, inhibiting phosphatase activity. A low-level of basal activity remains due to a small percentage of open, active conformation. A high-affinity peptide that carries a phosphotyrosine residue (pY) cannot fully occupy its cognate binding site on N-SH2 due to steric hindrance by the EF loop; however, the pY moiety can still bind. After spontaneous transition of SHP2–pY to its open conformation, the bound pY triggers conformational fluctuations (represented by broad cartoons) in several regions of N-SH2, disfavoring the re-closing of SHP2-pY and simultaneously opening up the N-SH2 specificity pocket to accommodate the phosphopeptide residues C-terminal to pY+3. The full binding of the phosphopeptide then stabilizes the N-SH2 loops in a conformation that precludes interaction with the PTP domain and hence maintains the accessibility of the catalytic site of the PTP domain. The SHP2–phosphopeptide complex is thus kept in its activated state for as long as the phosphopeptide remains bound to the N-SH2 domain. and are the rate constants for the transition between the closed and open conformations of SHP2 in the presence of bound pY. and are the rate constants for binding and unbinding of the C-terminal part of the peptide to the specificity pocket of N-SH2.