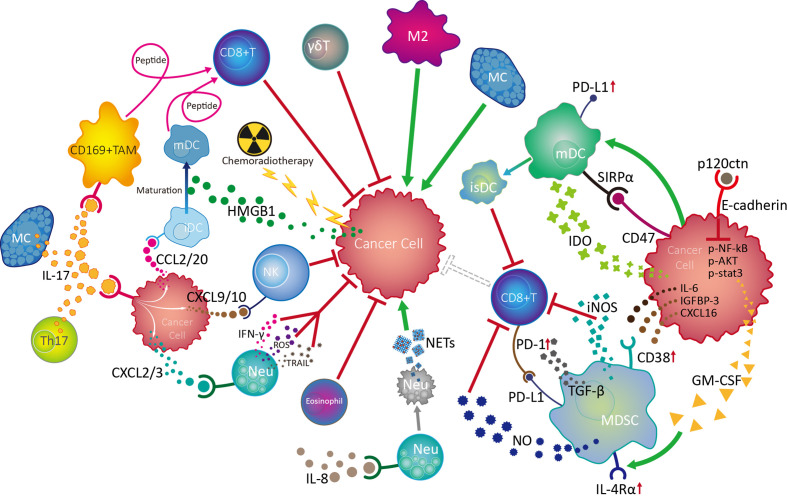
Figure 3.
Crosstalk and regulation of innate immune cells in the esophageal cancer tumor microenvironment. Among the multiple innate immune cells involved in esophageal cancer progression, NK and γδT cells are active in the front line of anti-tumor defences with their powerful cytotoxicity. Mature dendritic cells (mDC) play vital roles in antitumour responses by boosting the function of CD8+ effector T cells, while chemoradiotherapy can promote DC maturity by increasing HMGB1 levels in the tumor microenvironment (TME). IL-17 derived from Th17 cells and MCs can activate CD169+ tumor infiltrating macrophages (TAMs) and effector CD8+ T cells, as well as recruit NK cells, CD1a+ immature DCs (iDC) and neutrophils (Neu) into the TME by stimulating cancer cells to release various chemokines, thereby exerting antitumour effects. Although the mechanism is unknown, eosinophils are also involved in the anti-tumor process. On the contrary, neutrophils can also promote tumor development by inhibiting NK cell function in response to IL-8, as well as by forming NETs after tumor-induced apoptosis. MCs can promote tumor cell growth through the TK1/mitogenic kinin pathway. Importantly, tumor cells can escape from innate immune surveillance by promoting TAM progression to a suppressive M2 phenotype, which inhibits CD8+ T cell function by transforming DCs into an immunosuppressive phenotype (isDC) and recruiting myeloid-derived suppressor cells to inhibit the cytotoxic effects of CD8+ T and NK cells.