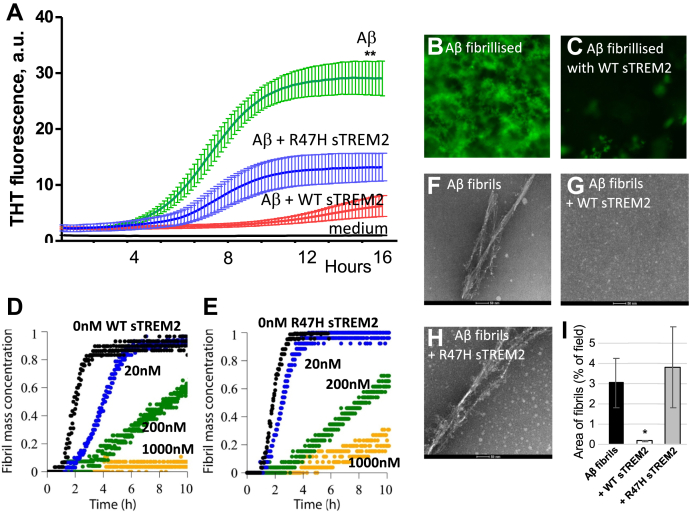
Figure 3.
Wild-type sTREM2 blocks Aβ fibrillization, but R47H inhibits less. A, 10 μM of monomeric Aβ was incubated at 30oC in DMEM/F12 with 10 μM thioflavin T (THT) ± 1 μM wild-type or R47H sTREM2, and the fluorescence followed over time. Means and SD of three separate experiments are shown. There was significant difference (p < 0.01, ∗∗) between the final fluorescence of the Aβ v Aβ + WT sTREM2 samples. At the end of the assay, (B) in the absence of sTREM2, and (C) presence of wild-type sTREM2, the bottom of the well was imaged using a fluorescence microscope with a ×40 objective. D, 2 μM of monomeric Aβ was incubated at 37 °C in phosphate buffer with 10 μM THT +0, 0.02, 0.2, or 1.0 μM wild-type sTREM2, and the fluorescence followed over time. E, 2 μM of monomeric Aβ was incubated at 37 °C in phosphate buffer with 10 μM THT +0, 0.02, 0.2, or 1.0 μM R47H sTREM2, and the fluorescence followed over time. Preformed Aβ fibrils were either (F) untreated, (G) treated with WT sTREM2, or (H) treated with R47H sTREM2 at molar ratios of 1:1 and TEM imaged after 2 h. I, quantification of the area of Aβ fibrils confirmed that WT sTREM2 decreased Aβ fibrils, and R47H sTREM2 had no effect. Images (F–H) and data (I) are reproduced in Figure S12 with those for 30 min and 24 h for comparison. Error bars represent SD. Statistical analysis was performed using one-way ANOVA followed by Bonferroni's multiple comparison test (n = 3–8, ∗p < 0.05 versus preformed Aβ fibril).