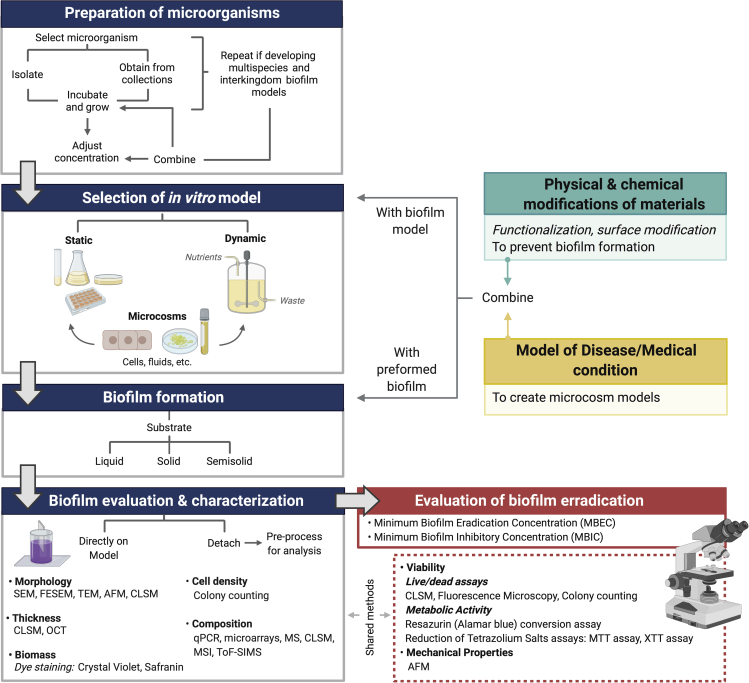
Figure 9.
Schematic depiction for the overall steps involved in the current standard in vitro protocols of biofilm formation
Blue heading boxes show the general methodology followed in studies of biofilm development; same procedure is utilized when investigating the efficacy of biofilm eradication strategies (red box). Moreover, if prevention of biofilm formation is the goal of the study (green box) or if microcosm models are required (yellow box), functionalization/modification of materials and development of the appropriate disease models are respectively needed. After this, the materials under evaluation and the disease models may be combined with the chosen in vitro model for biofilm formation or with a preformed biofilm. Evaluation and characterization of the biofilm may be done directly on the model or detachment may be required for processing and analysis, depending on the chosen methods. Representative examples of the methods and techniques used for characterizing biofilms are shown; some of them are also used to evaluate the effect of antibiofilm strategies. SEM, scanning electron microscopy; FESEM, field emission SEM; TEM, transmission electron microscopy; AFM, atomic force microscopy; OCT, optical coherence tomography; qPCR, quantitative real-time PCR; MS, mass spectrometry; MSI, MS imaging; ToF-SIMS, time-of-flight secondary ion MS.