Abstract
The future of coral reef ecosystems is under threat because vital reef-accreting species such as coralline algae are highly susceptible to ocean acidification. Although ocean acidification is known to reduce coralline algal growth rates, its direct effects on the development of coralline algal reproductive structures (conceptacles) is largely unknown. Furthermore, the long-term, multi-generational response of coralline algae to ocean acidification is extremely understudied. Here, we investigate how mean pH, pH variability and the pH regime experienced in their natural habitat affect coralline algal conceptacle abundance and size across six generations of exposure. We show that second-generation coralline algae exposed to ocean acidification treatments had conceptacle abundances 60% lower than those kept in present-day conditions, suggesting that conceptacle development is initially highly sensitive to ocean acidification. However, this negative effect of ocean acidification on conceptacle abundance disappears after three generations of exposure. Moreover, we show that this transgenerational acclimation of conceptacle development is not facilitated by a trade-off with reduced investment in growth, as higher conceptacle abundances are associated with crusts with faster growth rates. These results indicate that the potential reproductive output of coralline algae may be sustained under future ocean acidification.
Keywords: acclimation, conceptacles, coralline algae, multi-generational, ocean acidification, pH variability
1. Introduction
Ocean acidification is a threat to many marine organisms, with calcifying taxa and the ecosystems they support particularly at risk [1,2]. As a hotspot for marine biodiversity [3,4], and an annual source of billions of dollars to the global economy [5] coral reefs are one of the most valuable ecosystems threatened by ocean acidification. These ecosystems are vulnerable because framework-building corals and the coralline algae that cement this framework together may both be impaired by changes in seawater carbonate chemistry [1,2]. Furthermore, coralline algae are predicted to be highly susceptible to these altered conditions, as they precipitate one of the most soluble calcium carbonate polymorphs, high magnesium calcite [6,7]. Coralline algae are important to the functioning of coral reefs as they maintain the structural integrity of reefs by cementing them together, while also providing a number of ecosystem services such as facilitating coral larval settlement [8,9] and providing food for invertebrates [10]. Therefore, if coralline algal survival, growth or reproductive output is reduced by future ocean acidification, these services that are vital to the persistence of coral reefs could be lost. Previous research has demonstrated reduced calcification and growth in mature coralline algae under ocean acidification [11–13], as well as significant reductions in coralline algae at natural analogues of future ocean acidification [14,15]. Moreover, coralline algal recruitment and calcification during early life stages appear to be highly susceptible to reduced seawater pH lower than 7.91 as demonstrated through decreasing calcification rates and approximately 90% reduced recruitment rate (or success) [16–18]. If such large reductions in recruitment are realized under ocean acidification, coralline algal populations will inevitably experience significant declines, with severe negative consequences for coral reefs [19].
When assessing the impacts of environmental change on marine organisms it is important to consider timescales that match the time span organisms will have to respond to external changes, as previous studies have demonstrated differing responses under long and short term exposure [20,21]. Such considerations are also required as a host of transgenerational processes can act over multiple generations to improve fitness. For example, tropical reef fish are initially sensitive to ocean warming but can rapidly acclimate over two generations [22], while rock oyster larvae exposed to elevated pCO2 grew larger and developed faster when their parents also experienced elevated pCO2 [23]. When discussing generational improvements in performance it is important to define the use of terms such as acclimation or adaptation. Here, we use the term acclimation to refer to changes in performance driven by non-genetic plasticity in responses [22,24,25], and the term adaptation to refer to changes in performance driven by genetic change [26,27]. A recent study by Cornwall et al. [18] was the first to assess the response of a reef-accreting calcifier to ocean acidification across multiple (greater than two) generations. They report that although coralline algae are initially highly sensitive to ocean acidification, growth was no longer affected by ocean acidification after six generations of exposure. Yet, this novel result should be interpreted with caution, as increases in growth after multiple generations under ocean acidification may be at the expense of other processes such as reproduction. Similar trade-offs have been observed in coralline algae, where maintaining calcification rates under ocean acidification was coupled with harmful changes in skeletal ultrastructure [28,29], although such trade-offs are not always observed [30]. Therefore, while measuring growth is valuable, a multi-generational assessment of reproductive structures in response to ocean acidification will provide further insights into how coralline algae respond to ocean acidification.
The pH variability within any one habitat can dictate the sensitivity of resident calcifying species to ocean acidification. Exposure to variable pH regimes is common in shallow-water reef ecosystems where local metabolic activity and long water residence times combine to cause large diurnal oscillations in seawater chemistry [31,32]. Such variable pH regimes may mitigate the effects of ocean acidification on coralline algae by facilitating enhanced calcification rates during the day [13,33], by selecting for individuals with low night pH tolerance due to phenotypic plasticity [34,35] or natural transgenerational acclimation [25,36]. However, such hypothesized benefits are not always apparent, with roughly half of past studies indicating coralline algae respond negatively to greater pH variability [37], and that pre-exposure to low pH due to pH variability does not facilitate acclimation to ocean acidification [18,38–40]. Contrasting results such as these suggest the effect of pH variability on coralline algal responses to ocean acidification may be species or population specific. To date, the impacts of pH variability on the conceptacles of coralline algae from sites with vastly different pH regimes has not been assessed.
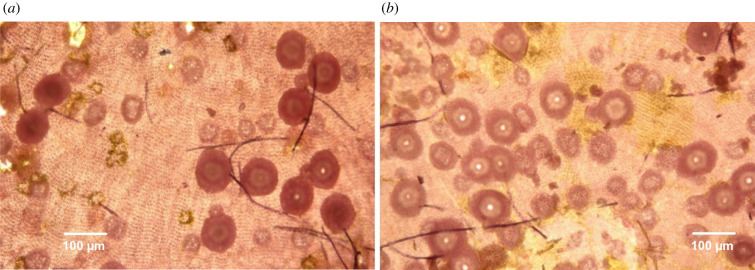
To understand the vulnerability of coralline algal recruitment and early life stages to ocean acidification, it would be ideal to isolate and investigate the various stages of reproduction and early life stage development. Coralline algae theoretically have a triphasic life cycle that allows reproduction via both sexual and asexual pathways. In coralline algae, like in many rhodophytes, the asexual phase may be the only one ever observed [41] and occurs through tetraspore-to-tetraspore-cycling, in which tetrasporophytes produce self-perpetuating diploid spores [42]. Additionally, tetrasporophytes have been observed to produce diploid bispores, which can also grow directly into tetrasporophytes [42]. In coralline algae, many reproductive structures, including the spore-producing sporangia, are housed within reproductive conceptacles found on the surface of intergenicula or crusts [42]. Thus, the development and abundance of these conceptacles (figure 1) have a key role in sustaining coralline algal populations and can be used as a proxy for reproductive output. However, it is important to note that measurements of conceptacle abundance do not exactly equal fecundity. Despite their importance, there has been limited research into the direct effects of ocean acidification on coralline algal conceptacles, with the single study conducted finding no impact of reduced pH (7.81, 7.60) on Pneophyllum sp. conceptacle numbers [43].
Figure 1.
(a,b) Images of the Hydrolithon reinboldii conceptacles quantified in this study. Shown are examples of the microscopic images used to quantify conceptacle abundance and diameter. (Online version in colour.)
Here, we investigate how reduced mean pH, differing levels of pH variability and the pH regime experienced by coralline algae in their natural habitat impact the abundance and size of conceptacles of the reef-building coralline algae Hydrolithon reinboldii, across six generations of exposure. We hypothesize that (i) coralline algal conceptacle abundance and size will decline under ocean acidification, (ii) prior exposure to low pH will not influence H. reinboldii's tolerance to ocean acidification, (iii) diurnal pH variability will not reduce the effect of ocean acidification and (iv) any impact of ocean acidification on H. reinboldii conceptacles will be reduced after multiple generations of exposure.
2. Material and methods
(a) . Treatment design and experimental set-up
Coralline algal rhodoliths of the species H. reinboldii were collected from two sites with contrasting pH regimes in the macrotidal Kimberley region of Australia in October 2016 (see electronic supplementary material, figure S1 for a map of these sites and electronic supplementary material, figure S2 for information on the pH regimes). See electronic supplementary material, Methods for site description and collection information. To investigate the effects of mean pH and different levels of pH variability on H. reinboldii four pH treatments were employed within aquaria. Two treatments had a mean pHT of 8.00, selected to represent the present-day mean surface seawater pHT detailed in ocean acidification studies best practices guide [44], and two treatments had a mean pHT of 7.70, designed to simulate potential future pH under ocean acidification (representative concentration pathway 8.5; 37). For the two treatments with the same mean pH, one was characterized by high pH variability and the other low pH variability, designed to emulate the pH regimes of Tallon and Shell Island, respectively. Thus, the four experimental pH treatments were denoted: (i) present-day low variability (8.00 mean pHT, mean daily range of 0.14), (ii) present-day high variability (8.00 mean pHT, mean daily range of 0.58), (iii) ocean acidification low variability (7.70 mean pHT, mean daily range of 0.20) and (iv) ocean acidification high variability (7.70 mean pHT, mean daily range of 0.89). electronic supplementary material, figure S3 highlights the pH regime of all four treatments and electronic supplementary material, table S1 displays the achieved pH for each of the four pH treatments used. Coralline algae collected from the two sites were divided among the four treatments in 48 experimental tanks, resulting in six independent experimental tanks for each of the eight pH treatment × site of origin combinations. See electronic supplementary material, Methods for full details of experimental set-up and design.
(b) . Multi-generational experiment
In this study only the conceptacles of coralline algae that recruited during the experiment were assessed, therefore, the generation 1 mature rhodoliths collected from the wild (F0), were used solely as source populations. The juvenile coralline algae assessed in this study are the same as those studied in Cornwall et al. [18]. While in the experimental tanks the generation 1 wild coralline algae released spores which went on to form recruits on the inside walls of the tanks. After 78 days, these generation 2 coralline algal recruits were visible on the walls of the experimental tank. Mature wild individuals were removed from the tanks on day 128 and two 4.5 cm × 5.0 cm (length × width) sections were cut out of each tank. These two sections of each tank were primarily cut from the area near the water outflow where possible, as this area contained the highest cover of coralline algal recruits. These plates were then placed into completely new tanks (in the same treatment as the previous generation), allowing for assessment of this second generation. Within these new experimental tanks second-generation algae formed conceptacles and released spores, which again settled onto the walls of the tank. Two 4.5 cm × 5.0 cm sections containing generation 3 recruits were cut from the experimental tank and placed into a new experimental tank (see electronic supplementary material, figure S4 for schematic of this experimental approach). This process was repeated every 41–51 days, allowing five generations (generation numbers 2–6) of plate-based coralline algal conceptacles to be studied. Coralline algal recruits across all generations possessed uniporate conceptacles, containing multiple tetrasporangial spores which are characteristic of H. reinboldii [41], and suggests each new generation was a result of asexual reproduction [45]. Contamination by foreign spores from local environments was unlikely due to the consistent times to visible recruitment across generations, the absence of coralline algae recruiting in the header tanks and the observation that all recruits had similar anatomical features [41]. To investigate whether any observed differences across generation were due to an altered response to treatments or an interaction between altered response to treatments and laboratory effects [46], a reciprocal transplant experiment was conducted. For full details of this reciprocal transplant experiment, see electronic supplementary material, Methods.
(c) . Conceptacle measurements
At the end of each generation, the coralline algae plates were removed from the tanks and stored in silica gel for up to 3 years, before three random locations on each plate were blindly selected and photographed using a compound microscope for analysis. For each plate conceptacle abundance was measured by counting all the visible conceptacles in the area of analysis. Individual conceptacle diameters were measured as the distance between the two most distant points of each conceptacle. For full details of microscopic conceptacle imaging and analysis see electronic supplementary material, Methods.
(d) . Growth and recruitment measurements
Multi-generational growth and total recruit area of the same coralline algae populations were investigated in relation to the conceptacle abundances measured in this study. Full methods and data are reported in Cornwall et al. [18], but see electronic supplementary material, Methods for details of these measurements and their comparison with conceptacle abundances.
(e) . Statistical analysis
Conceptacle abundance per plate and mean conceptacle diameter per plate was analysed using generalized linear mixed models with generation, mean treatment pH, pH variability and site of origin as fixed factors and header tank and water bath as random effects. As data were not normally distributed a Poisson distribution was specified for conceptacle abundances and a gamma distribution was specified for conceptacle diameter measurements. For the reciprocal transplant experiment, both conceptacle abundance and diameter were assessed using the same model structure, except that generation and mean treatment pH factors were replaced with the factors; generation 2–6 mean treatment pH and transplant treatment mean pH. Following the reciprocal transplant experiment, Wilcoxon rank-sum tests were used to test for pairwise differences in conceptacle abundances between the different generation 2–6 and transplant treatment combinations.
To allow for comparison with growth and recruitment data, mean conceptacle abundances per plate were averaged for each experiment tank (total of 48 per generation). The effect of conceptacle abundance on the total recruit area of the following generation, as well as the influence of treatment mean pH was assessed using generalized linear mixed models with conceptacle abundance and mean treatment pH as fixed factors. A quasi-Poisson model was used to account for the non-normality of data and overdispersion greater than 1. The same model was used to investigate whether changes in growth rates impacted the abundance of conceptacles, however, in this model growth and mean pH were specified as fixed factors. Assumptions of all models were checked via visual inspection of standard model diagnostic plots. All analyses were performed in R v. 3.6.1.
3. Results
(a) . Conceptacle abundance
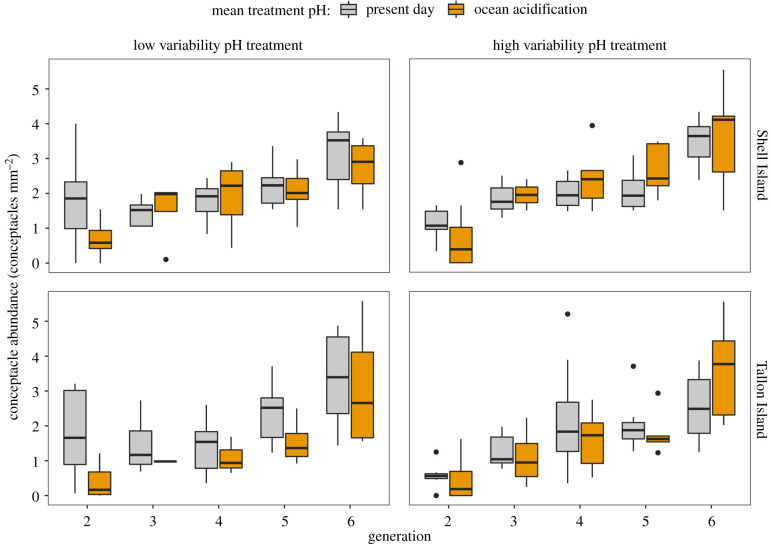
The abundance of conceptacles varied with generation (F = 206.882, p < 0.001), treatment mean pH (F = 8.241, p = 0.004) and coralline algae site of origin (F = 5.354, p = 0.022) (figure 2). No interactive effects between the site of origin, mean treatment pH and level of pH variability were observed, therefore, abundance and size data from different populations and pH variability treatments were grouped for subsequent analyses of mean treatment pH and generational effects (electronic supplementary material, figure S5). Coralline algae exposed to ocean acidification treatments for two generations had a mean conceptacle abundance that was 60% lower than coralline algae kept in present-day treatments (electronic supplementary material, figure S5). (Mean conceptacle abundances, standard error and n for each generation by treatment combination are detailed in electronic supplementary material, table S2.) However, after three generations of exposure the reduction in mean conceptacle abundances under ocean acidification declined to 7% (electronic supplementary material, figure S5). This differing effect of treatment mean pH across generations is supported by a significant interaction between generation and mean pH (F = 8.204, p = 0.005).
Figure 2.
H. reinboldii conceptacle abundance after exposure to present-day (mean pH 8.00) and ocean acidification (mean pH 7.70) treatments for between two and six generations. Figures are split by the site of origin (Shell or Tallon Island) and variability of pH treatment (low or high). Median, 25% and 75% quartiles are presented. (Online version in colour.)
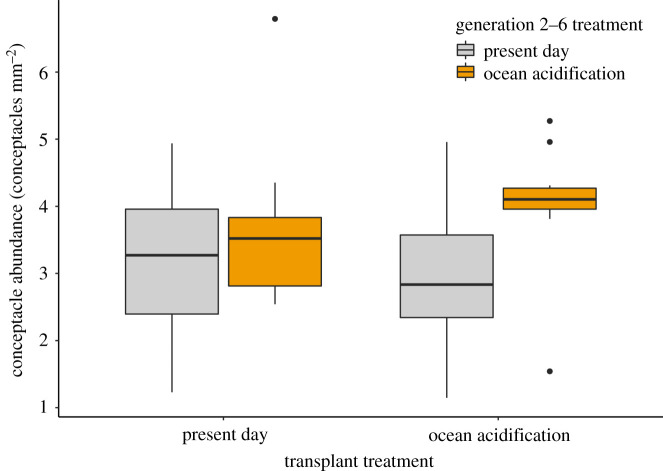
During the reciprocal transplant experiment, coralline algae exposed to ocean acidification treatments throughout generations 2–6 had higher conceptacle abundances than those in present-day treatments in generations 2–6 (F = 7.797, p = 0.012) (figure 3). However, transplant treatment (F = 0.059, p = 0.810) and the interaction between generation 2–6 treatment and transplant treatment (F = 0.303, p = 0.589) did not affect conceptacle abundance. Populations exposed to ocean acidification treatments for seven generations had a mean conceptacle abundance of 4.04 conceptacles mm−2, whereas those in present-day treatments throughout generations 2–6, had a mean conceptacle abundance of 3.06 conceptacles mm−2 after being transplanted to ocean acidification treatments in generation 7. This represents a 25% decline in conceptacle abundance in populations transplanted to ocean acidification treatments compared with those in ocean acidification treatments for seven generations. This difference is demonstrated by a significant pairwise difference between conceptacle abundances in these two reciprocal transplant groups (W = 38.5, p = 0.024). Mean conceptacle abundance, standard error and n for each generation 2–6 treatment by transplant treatment combination is detailed in electronic supplementary material, table S3.
Figure 3.
Results from reciprocal transplant experiment. H. reinboldii conceptacle abundance after seven generations of exposure to present-day and ocean acidification treatments (same generation 2–6 treatment and transplant treatment), and those transplanted to novel treatments in generation 7 (different generation 2–6 and transplant treatment). Responses to low and high variability pH treatments are combined. Median, 25% and 75% quartiles are displayed. (Online version in colour.)
(b) . Conceptacle abundance versus growth and recruitment
In each generation an increase in mean coralline algal growth per tank has a positive effect on the mean conceptacle abundance of the same coralline algae (electronic supplementary material, figure S6). Increasing conceptacle abundance with increasing growth rates are evidence of this (F = 110.937 p < 0.001). The relationship between growth and conceptacle abundance is not affected by mean treatment pH (F = 0.006, p = 0.937) or generation (F = 1.958, p = 0.163). As the conceptacle abundance of the plate-based coralline algae increases, the total recruit area (of the next generation) derived from that population also increases (F = 39.671, p < 0.001) (electronic supplementary material, figure S7). Mean treatment pH (F = 18.848, p < 0.001) and generation (F = 7.610, p = 0.006) have a significant effect on total recruit area (F = 18.848, p < 0.001). The relationship between conceptacle abundance and next-generation recruitment is not affected by mean treatment pH (F = 0.006, p = 0.937) yet it is affected by generation (F = 5.100, p = 0.0250).
(c) . Conceptacle size
Conceptacle diameter increased with generation (F = 14.099, p < 0.001, d.f. = 1) (electronic supplementary material, figure S8), however, diameter did not vary with mean treatment pH, site of origin or level of pH variability (electronic supplementary material, table S7 and figure S9). The reciprocal transplant experiment showed no effect of generation 2–6 treatment, transplant treatment or any interaction between the two on diameter (electronic supplementary material, figure S10).
4. Discussion
Here, we demonstrate that coralline algal conceptacle abundance is initially impacted by ocean acidification, but conceptacle size is not. This result indicates that the initial formation of conceptacles, rather than their later development is impaired by altered carbonate chemistry. However, as the first study to assess the impact of ocean acidification on conceptacles across multiple generations, this study also provides evidence that coralline algae may be more robust to the effects of ocean acidification than widely predicted, as from the third generation onwards there is no impact of ocean acidification on conceptacles (figure 2). Such an observation indicates that coralline algal conceptacles will continue to develop in a more acidic ocean. This is important as conceptacles are vital reproductive structures, without which the production of spores and subsequent production of next-generation recruits would not be possible. Thus, if conceptacles were impacted by future ocean acidification it would have severe consequences for the persistence and proliferation of coralline algal populations. This ability to maintain conceptacle abundances and size under ocean acidification will also be beneficial to other coral reefs organisms that are threatened by environmental change [1,2]. For example, continuing spore production and the formation of new coralline algal crusts will provide substrate for future coral larval settlement [8,9] and will also aid in binding calcium carbonate reef structures together, as they are increasingly weakened by ocean acidification [7,28]. Therefore, through such downstream effects, the maintenance of coralline algal conceptacles under ocean acidification could be vital to the future persistence of coral reefs.
We consider it likely that the reduced effect of ocean acidification across generations observed in this study is due to non-genetic acclimation, as coralline algae across all generations possessed features that are indicative of asexual reproduction [45]. However, we cannot definitively state that the changes observed here were not driven by selective genetic adaptation across generations. The transgenerational acclimation of coralline algal conceptacle abundances to ocean acidification observed here follows the trend observed for growth rates [18]. Moreover, the positive relationship between conceptacle abundances and growth rates (electronic supplementary material, figure S6) across all generations and treatments demonstrates that trade-offs between these two processes are not underlying the multi-generational acclimation of conceptacle abundance and growth to ocean acidification. For example, if such a trade-off was driving this acclimation you would expect to see a negative relationship between growth and conceptacle abundance within the later ‘acclimated’ generations. This observation that multi-generational acclimation is observed for conceptacle abundance and growth [18] further indicates that coralline algae are capable of acclimating to ocean acidification. However, despite the overall similarity in responses, acclimation of growth rates to ocean acidification were stepwise/linear in nature (reported in Cornwall et al. [18]), and therefore not as rapid as the acclimation of conceptacles. While the acclimation of conceptacle formation observed here is deemed rapid compared with growth, similar rapid transgenerational acclimation has been observed in differing marine taxa. For example, the reproductive output of marine invertebrates such as polychaetes [47] and copepods [48] is initially impaired under ocean acidification; however, this effect disappears by the third generation, as the reproductive output of populations exposed to ocean acidification does not differ from controls. Additionally, transgenerational acclimation of growth rates to ocean acidification has been observed within three generations of exposure in calcifying organisms such as oysters [49] and clams [24], suggesting various biological processes are capable of rapid acclimation to ocean acidification. However, our results and those of Cornwall et al. [18] suggest that different processes may exhibit contrasting acclimation times.
The varying rates of acclimation of different biological processes in coralline algae are likely driven by the physiological mechanisms underpinning these processes. Here, we propose two mechanisms that may have driven the faster multi-generational acclimation of conceptacle development, compared with growth [18]. Firstly, linear extension of coralline algae is dictated by both growth of organic tissue and calcification of the cell walls. The latter is impacted by factors such as the control of calcification site chemistry and the production of organic matrices, both of which may be disrupted by ocean acidification [50,51]. Thus, the complexity of calcification and its dependence on specific chemical conditions may represent a physiochemical bottleneck to the rate at which calcification, and therefore growth can acclimatize to ocean acidification. By contrast, the primarily uncalcified [52,53] conceptacles are not subject to the same chemical constraints, potentially facilitating faster acclimation. An alternate and not mutually exclusive explanation is that coralline algae prioritize energetic investment in reproduction over calcification. Energetic trade-offs between reproduction and other processes such as growth commonly occur in nature [54,55], and are enhanced due to increased maintenance costs under stressful conditions [56]. Therefore, rapid acclimation of reproductive conceptacles may come from prioritizing energetic investment in reproduction, as ensuring the production of offspring under stress is of the greatest benefit to Darwinian fitness. Similar multi-generational trends in resource allocation towards reproduction under ocean acidification have also been observed in copepods [57]. Here, our novel comparison of acclimatory responses (growth rates versus conceptacle development), indicates that the persistence of populations under ocean acidification will be constrained by the biological process that undergoes the slowest rate of acclimation.
The reduction in second-generation conceptacle abundances under ocean acidification mirror the widely reported negative impacts of ocean acidification on the calcification, growth and recruitment of first and second-generation coralline algae [11,16,58]. This initial reduction in conceptacle abundance observed here, highlights reduced conceptacle formation as one potential mechanism driving these previously reported declines in coralline algae recruitment under ocean acidification [16,17]. However, in contrast to the initial negative effect observed here, Ordoñez et al. [43] found that conceptacle numbers in Pneophyllum sp. coralline algae were not reduced when exposed to medium (pH 7.81) or low (pH 7.60) pH treatments. Such a result is unexpected, as these populations were subject to neither transgenerational nor developmental exposure to low pH conditions, as they recruited into experimental conditions from the wild. However, species-specific responses to ocean acidification have been documented in coralline algae [37,59], therefore, this opposing result may be due to differences between the thin-crusted Pneophyllum sp. studied by Ordoñez et al. [43], and the thick-crusted H. reinboldii assessed here. Evidence of such species effects are displayed in Ordoñez et al. [43] as they observed a significant decline in the relative density of thick-crusted Porolithon onkodes under high pCO2, but not Pneophyllum sp., suggesting that morphology can influence response to ocean acidification. Alternatively, these populations may have had natural resilience to this magnitude of ocean acidification, and further research is required to elucidate which factors could be driving such differences.
The rapid acclimation of reproductive conceptacles observed here is not translated to rapid increases in the final recruit area of the next generation, as recruit area acclimation was slower and stepwise [18]. Therefore, although the trend of increasing recruit area with increasing conceptacle abundance (electronic supplementary material, figure S7) suggests that higher conceptacle abundances may aid in buffering the impacts of ocean acidification on recruitment, it is likely that final recruitment will be determined by the cumulative impact of ocean acidification on reproductive structure formation, spore settlement and early development processes. For example, coralline algae display delayed and weakened spore attachment [60], as well as reduced spore germination [61] in response to ocean acidification, potentially highlighting the later sporulate stage as a bottleneck in final recruitment.
Our results also demonstrate that pH variability does not modify the impact of ocean acidification on coralline algal conceptacles. This is shown as coralline algae grown in both high variability and low variability pH treatments were not differently affected by the mean pH corresponding to ocean acidification. This lack of effect of pH variability is at odds with some past literature, where pH variability has been shown to reduce the impact of ocean acidification [33,38], while it can also enhance the negative impacts of ocean acidification [37]. These contrasting and inconsistent effects of pH variability demonstrate that its interaction with future ocean acidification will be complex and can potentially elicit a full range of effects from negative to positive, with the lack of effect observed here in between the gamut of responses. As previous studies have highlighted that the influence of pH variability varies among species [20,62], it appears that the effect of ocean acidification on H. reinboldii is not dictated by pH variability as the calcification and photosynthesis of mature rhodoliths [40], the growth rates and recruitment of juveniles [18] and the size and abundance of juvenile conceptacles are all unaffected.
The effect of ocean acidification on coralline algal conceptacles is not influenced by the pH variability experienced by the population in their natural environment here. This was hypothesized a priori, as similar responses were observed with adult calcification rates [40] and juvenile growth rate [18]. Nevertheless, this result contrasts with the often-posed hypothesis in the literature that environments with naturally high variations in an abiotic factor, will increase the resistance of resident organisms to future mean changes in that abiotic factor [37]. Though this trend has recently been observed in corals from habitats with greater thermal variability [63,64], mean conditions may be as important or more important than variability [65]. For example, mean pH is higher at Tallon Island, while pH variability is greater than Shell Island. Therefore, both could be acting against each other in conditioning coralline algal responses to low pH. Our results suggest a similar priming effect of mean pH, as three generations of exposure to reduced mean pH was enough to eliminate the effects of reduced mean pH on the conceptacles of later generations.
Overall, this study demonstrates that the abundance of coralline algal conceptacles is greatly reduced in second-generation coralline algae reared under simulated ocean acidification conditions. However, in just three generations of exposure to these same ocean acidification conditions coralline algal reproductive structures can rapidly acclimate, as we observe no effects of ocean acidification beyond the second generation. This acclimation is much faster than that previously reported for growth rates, as growth took six generations to acclimate. Despite differing rates of acclimation, the acclimation of conceptacle development observed here is not facilitated by reduced growth, as both processes acclimate together across all generations. Therefore, this study further supports the possibility that coralline algae could acclimate to the effects of ocean acidification. As multiple studies have reported the ability of marine organisms to acclimate to environmental change, our observation that acclimation rates vary for different biological processes introduces a new consideration for studies assessing how marine organisms will respond to future environmental change.
Supplementary Material
Ethics
All local regulations and permit requirements were followed during this study.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.05qfttf27 [66].
Authors' contributions
B.M.: data curation, formal analysis, investigation, methodology, validation, visualization, writing original draft, writing-review and editing; S.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing-review and editing; M.B.: data curation, investigation, methodology, writing-review and editing; A.C.: data curation, investigation, methodology, writing-review and editing; A.P.: data curation, investigation, methodology, writing-review and editing; E.L.: data curation, investigation, methodology, writing-review and editing; F.P.: data curation, investigation, methodology, writing-review and editing; M.T.M.: conceptualization, funding acquisition, project administration, resources, supervision, writing-review and editing; C.E.C.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing-review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by an ARC Centre of Excellence for Coral Reef Studies grant (no. CE140100020) awarded to M.T.M., an ARC Discovery Early Career Researcher Award (no. DE160100668) awarded to S.C. and an ARC Laureate Fellowship (grant no. LF120100049) awarded to M.T.M. C.E.C. was also supported by a Rutherford Discovery Fellowship from The Royal Society of New Zealand Te Apārangi (no. RDF-VUW1701).
References
- 1.Chan NCS, Connolly SR. 2013. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 19, 282-290. ( 10.1111/gcb.12011) [DOI] [PubMed] [Google Scholar]
- 2.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884-1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouillot D, et al. 2014. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13 757-13 762. ( 10.1073/pnas.1317625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts CM, et al. 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280-1284. ( 10.1126/science.1067728) [DOI] [PubMed] [Google Scholar]
- 5.O'Mahoney J, Simes R, Redhill D, Heaton K, Atkinson C, Hayward E, Nguyen M. 2017. At what price? The economic, social and icon value of the Great Barrier Reef. Deloitte Access Economics. See https://elibrary.gbrmpa.gov.au/jspui/bitstream/11017/3205/1/deloitte-au-economics-great-barrier-reef-230617.pdf.
- 6.Busenberg E, Plummer LN. 1989. Thermodynamics of magnesian calcite solid-solutions at 25°C and 1 atm total pressure. Geochim. Cosmochim. Acta 53, 1189-1208. ( 10.1016/0016-7037(89)90056-2) [DOI] [Google Scholar]
- 7.Ries JB. 2011. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 403, 54-64. ( 10.1016/j.jembe.2011.04.006) [DOI] [Google Scholar]
- 8.Price N. 2010. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747-758. ( 10.1007/s00442-010-1578-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Leary JK, Potts DC, Braga JC, McClanahan TR. 2012. Indirect consequences of fishing: reduction of coralline algae suppresses juvenile coral abundance. Coral Reefs 31, 547-559. ( 10.1007/s00338-012-0872-5) [DOI] [Google Scholar]
- 10.Kawamura T. 1998. A review of feeding and growth of postlarval abalone. J. Shellfish Res. 17, 615-625. [Google Scholar]
- 11.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442-17 446. ( 10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473-483. ( 10.1007/s00338-008-0380-9) [DOI] [Google Scholar]
- 13.Semesi IS, Kangwe J, Björk M. 2009. Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp. (Rhodophyta). Estuar. Coast. Shelf Sci. 84, 337-341. ( 10.1016/j.ecss.2009.03.038) [DOI] [Google Scholar]
- 14.Fabricius KE, Kluibenschedl A, Harrington L, Noonan S, De'Ath G. 2015. In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Sci. Rep. 5, 1-7. ( 10.1038/srep09537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia M-C. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96-99. ( 10.1038/nature07051) [DOI] [PubMed] [Google Scholar]
- 16.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, MacKenzie FT. 2008. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114-117. ( 10.1038/ngeo100) [DOI] [Google Scholar]
- 17.Crook ED, Kroeker KJ, Potts DC, Rebolledo-Vieyra M, Hernandez-Terrones LM, Paytan A. 2016. Recruitment and succession in a tropical benthic community in response to in-situ ocean acidification. PLoS ONE 11, 1-16. ( 10.1371/journal.pone.0146707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornwall CE, Comeau S, DeCarlo TM, Larcombe E, Moore B, Giltrow K, Puerzer F, D'Alexis Q, McCulloch MT. 2020. A coralline alga gains tolerance to ocean acidification over multiple generations of exposure. Nat. Clim. Change 10, 143-146. ( 10.1038/s41558-019-0681-8) [DOI] [Google Scholar]
- 19.Fabricius KE, Noonan SHC, Abrego D, Harrington L, De'Ath G. 2017. Low recruitment due to altered settlement substrata as primary constraint for coral communities under ocean acidification. Proc. R. Soc. B 284, 20171536. ( 10.1098/rspb.2017.1536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy SJ, Kamenos NA. 2015. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 51, 6-24. ( 10.1111/jpy.12262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sordo L, Santos R, Barrote I, Silva J. 2018. High CO2 decreases the long-term resilience of the free-living coralline algae Phymatolithon lusitanicum. Ecol. Evol. 8, 4781-4792. ( 10.1002/ece3.4020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donelson JM, Munday PL, McCormick MI, Pitcher CR. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30-32. ( 10.1038/nclimate1323) [DOI] [Google Scholar]
- 23.Parker LM, Ross PM, O'connor WA, Borysko L, Raftos DA, Pörtner H. 2012. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Change Biol. 18, 82-92. ( 10.1111/j.1365-2486.2011.02520.x) [DOI] [Google Scholar]
- 24.Zhao L, Yang F, Milano S, Han T, Walliser EO, Schöne BR. 2018. Transgenerational acclimation to seawater acidification in the Manila clam Ruditapes philippinarum: preferential uptake of metabolic carbon. Sci. Total Environ. 627, 95-103. ( 10.1016/j.scitotenv.2018.01.225) [DOI] [PubMed] [Google Scholar]
- 25.Goncalves P, Anderson K, Thompson EL, Melwani A, Parker LM, Ross PM, Raftos DA. 2016. Rapid transcriptional acclimation following transgenerational exposure of oysters to ocean acidification. Mol. Ecol. 25, 4836-4849. ( 10.1111/mec.13808) [DOI] [PubMed] [Google Scholar]
- 26.Sunday JM, Calosi P, Dupont S, Munday PL, Stillman JH, Reusch TBH. 2014. Evolution in an acidifying ocean. Trends Ecol. Evol. 29, 117-125. ( 10.1016/j.tree.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 27.Lohbeck KT, Riebesell U, Reusch TBH. 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346-351. ( 10.1038/ngeo1441) [DOI] [Google Scholar]
- 28.Ragazzola F, Foster LC, Form A, Anderson PSL, Hansteen TH, Fietzke J. 2012. Ocean acidification weakens the structural integrity of coralline algae. Glob. Change Biol. 18, 2804-2812. ( 10.1111/j.1365-2486.2012.02756.x) [DOI] [PubMed] [Google Scholar]
- 29.McCoy SJ, Ragazzola F. 2014. Skeletal trade-offs in coralline algae in response to ocean acidification. Nat. Clim. Change 4, 719-723. ( 10.1038/nclimate2273) [DOI] [Google Scholar]
- 30.Kamenos NA, Perna G, Gambi MC, Micheli F, Kroeker KJ. 2016. Coralline algae in a naturally acidified ecosystem persist by maintaining control of skeletal mineralogy and size. Proc. R. Soc. B 283, 20161159. ( 10.1098/rspb.2016.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthony KRN, Kleypas JA, Gattuso JP. 2011. Coral reefs modify their seawater carbon chemistry — implications for impacts of ocean acidification. Glob. Change Biol. 17, 3655-3666. ( 10.1111/j.1365-2486.2011.02510.x) [DOI] [Google Scholar]
- 32.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983. ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller JD, Pansch C, Sawall Y. 2017. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceanogr. 63, 3-21. ( 10.1002/lno.10608) [DOI] [Google Scholar]
- 34.Schaum CE, Collins S. 2014. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B 281, 20141486. ( 10.1098/rspb.2014.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas CA, Lagos NA, Lardies MA, Duarte C, Manríquez PH, Aguilera VM, Broitman B, Widdicombe S, Dupont S. 2017. Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 1, 1-7. ( 10.1038/s41559-017-0084) [DOI] [PubMed] [Google Scholar]
- 36.Schunter C, Welch MJ, Ryu T, Zhang H, Berumen ML, Nilsson GE, Munday PL, Ravasi T. 2016. Molecular signatures of transgenerational response to ocean acidification in a species of reef fish. Nat. Clim. Change 6, 1014-1018. ( 10.1038/nclimate3087) [DOI] [Google Scholar]
- 37.Rivest EB, Comeau S, Cornwall CE. 2017. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Change Rep. 3, 271-281. ( 10.1007/s40641-017-0082-x) [DOI] [Google Scholar]
- 38.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Mar. Ecol. Prog. Ser. 501, 99-111. ( 10.3354/meps10690) [DOI] [Google Scholar]
- 39.Johnson MD, Moriarty VW, Carpenter RC. 2014. Acclimatization of the crustose coralline alga Porolithon onkodes to variable pCO2. PLoS ONE 9, e87678. ( 10.1371/journal.pone.0087678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornwall CE, Comeau S, DeCarlo TM, Moore B, D'Alexis Q, McCulloch MT. 2018. Resistance of corals and coralline algae to ocean acidification: physiological control of calcification under natural pH variability. Proc. R. Soc. B 285, 20181168. ( 10.1098/rspb.2018.1168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend RA, Huisman JM. 2018. Algae of Australia: marine benthic algae of North-Western Australia. Melbourne, Australia: CSIRO.
- 42.Johansen HW. 1981. Coralline algae: a first synthesis. Boca Raton, FL: CRC Press. [Google Scholar]
- 43.Ordoñez A, Doropoulos C, Diaz-Pulido G. 2014. Effects of ocean acidification on population dynamics and community structure of crustose coralline algae. Biol. Bull. 226, 255-268. ( 10.1086/BBLv226n3p255) [DOI] [PubMed] [Google Scholar]
- 44.Riebesell U, Fabry VJ, Hansson L, Gattuso J-P. 2011. Guide to best practices for ocean acidification research and data reporting. Luxembourg City, Luxembourg: Office for Official Publications of the European Communities.
- 45.Harvey A. 2005. Coralline algae of central New Zealand: an identification guide to common'crustose'species. NIWA Inf. Ser. 57, 1-145. [Google Scholar]
- 46.Torda G, et al. 2017. Rapid adaptive responses to climate change in corals. Nat. Clim. Change 7, 627-636. ( 10.1038/nclimate3374) [DOI] [Google Scholar]
- 47.Rodríguez-Romero A, Jarrold MD, Massamba-N'Siala G, Spicer JI, Calosi P. 2016. Multi-generational responses of a marine polychaete to a rapid change in seawater pCO2. Evol. Appl. 9, 1082-1095. ( 10.1111/eva.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thor P, Dupont S. 2015. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob. Change Biol. 21, 2261-2271. ( 10.1111/gcb.12815) [DOI] [PubMed] [Google Scholar]
- 49.Parker LM, O'Connor WA, Raftos DA, Pörtner HO, Ross PM. 2015. Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE 10, 1-19. ( 10.1371/journal.pone.0132276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornwall CE, Comeau S, McCulloch MT. 2017. Coralline algae elevate pH at the site of calcification under ocean acidification. Glob. Change Biol. 23, 4245-4256. ( 10.1111/gcb.13673) [DOI] [PubMed] [Google Scholar]
- 51.Allison N, Cole C, Hintz C, Hintz K, Rae J, Finch A. 2018. The effect of ocean acidification on tropical coral calcification: insights from calcification fluid DIC chemistry. Chem. Geol. 497, 162-169. ( 10.1016/j.chemgeo.2018.09.004) [DOI] [Google Scholar]
- 52.Mu X, Riding R. 1999. Skeletal ultrastructure of the calcified red alga Galaxaura oblongata, Hainan Island, China. Rev. Palaeobot. Palynol. 104, 205-212. ( 10.1016/S0034-6667(98)00061-X) [DOI] [Google Scholar]
- 53.Adey WH, Halfar J, Williams B. 2013. The coralline genus Clathromorphum Foslie emend. Adey: biological, physiological, and ecological factors controlling carbonate production in an arctic–subarctic climate archive. Smithsonian Contr. Mar. Sci. 40, 1–41. [Google Scholar]
- 54.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95-126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 55.Harshman LG, Zera AJ. 2007. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80-86. ( 10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 56.Jager T, Ravagnan E, Dupont S. 2016. Near-future ocean acidification impacts maintenance costs in sea-urchin larvae: identification of stress factors and tipping points using a DEB modelling approach. J. Exp. Mar. Biol. Ecol. 474, 11-17. ( 10.1016/j.jembe.2015.09.016) [DOI] [Google Scholar]
- 57.Fitzer SC, Caldwell GS, Close AJ, Clare AS, Upstill-Goddard RC, Bentley MG. 2012. Ocean acidification induces multi-generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. J. Exp. Mar. Biol. Ecol 418–419, 30-36. ( 10.1016/j.jembe.2012.03.009) [DOI] [Google Scholar]
- 58.Johnson MD, Carpenter RC. 2012. Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. J. Exp. Mar. Biol. Ecol 434–435, 94-101. ( 10.1016/j.jembe.2012.08.005) [DOI] [Google Scholar]
- 59.Comeau S, Carpenter RC, Nojiri Y, Putnam HM, Sakai K, Edmunds PJ. 2014. Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc. R. Soc. B 281, 20141339. ( 10.1098/rspb.2014.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guenther R, Miklasz K, Carrington E, Martone PT. 2017. Macroalgal spore dysfunction: ocean acidification delays and weakens adhesion. J. Phycol. 54, 153-158. ( 10.1111/jpy.12614) [DOI] [PubMed] [Google Scholar]
- 61.Ordoñez A, Kennedy EV, Diaz-Pulido G. 2017. Reduced spore germination explains sensitivity of reef-building algae to climate change stressors. PLoS ONE 12, 1-19. ( 10.1371/journal.pone.0189122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noisette F, Egilsdottir H, Davoult D, Martin S. 2013. Physiological responses of three temperate coralline algae from contrasting habitats to near-future ocean acidification. J. Exp. Mar. Biol. Ecol. 448, 179-187. ( 10.1016/j.jembe.2013.07.006) [DOI] [Google Scholar]
- 63.Oliver TA, Palumbi SR. 2011. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429-440. ( 10.1007/s00338-011-0721-y) [DOI] [Google Scholar]
- 64.Schoepf V, Stat M, Falter JL, Mcculloch MT. 2015. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 1-14. ( 10.1038/srep17639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivest EB, Chen C-S, Fan T-Y, Li H-H, Hofmann GE. 2017. Lipid consumption in coral larvae differs among sites: a consideration of environmental history in a global ocean change scenario. Proc. R. Soc. B 284, 20162825. ( 10.1098/rspb.2016.2825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore B, Comeau S, Bekaert M, Cossais A, Purdy A, Larcombe E, Puerzer F, McCulloch MT, Cornwall CE. 2021. Data from: Rapid multi-generational acclimation of coralline algal reproductive structures to ocean acidification. Dryad Digital Respository. ( 10.5061/dryad.05qfttf27) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moore B, Comeau S, Bekaert M, Cossais A, Purdy A, Larcombe E, Puerzer F, McCulloch MT, Cornwall CE. 2021. Data from: Rapid multi-generational acclimation of coralline algal reproductive structures to ocean acidification. Dryad Digital Respository. ( 10.5061/dryad.05qfttf27) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.05qfttf27 [66].