Figure 1.
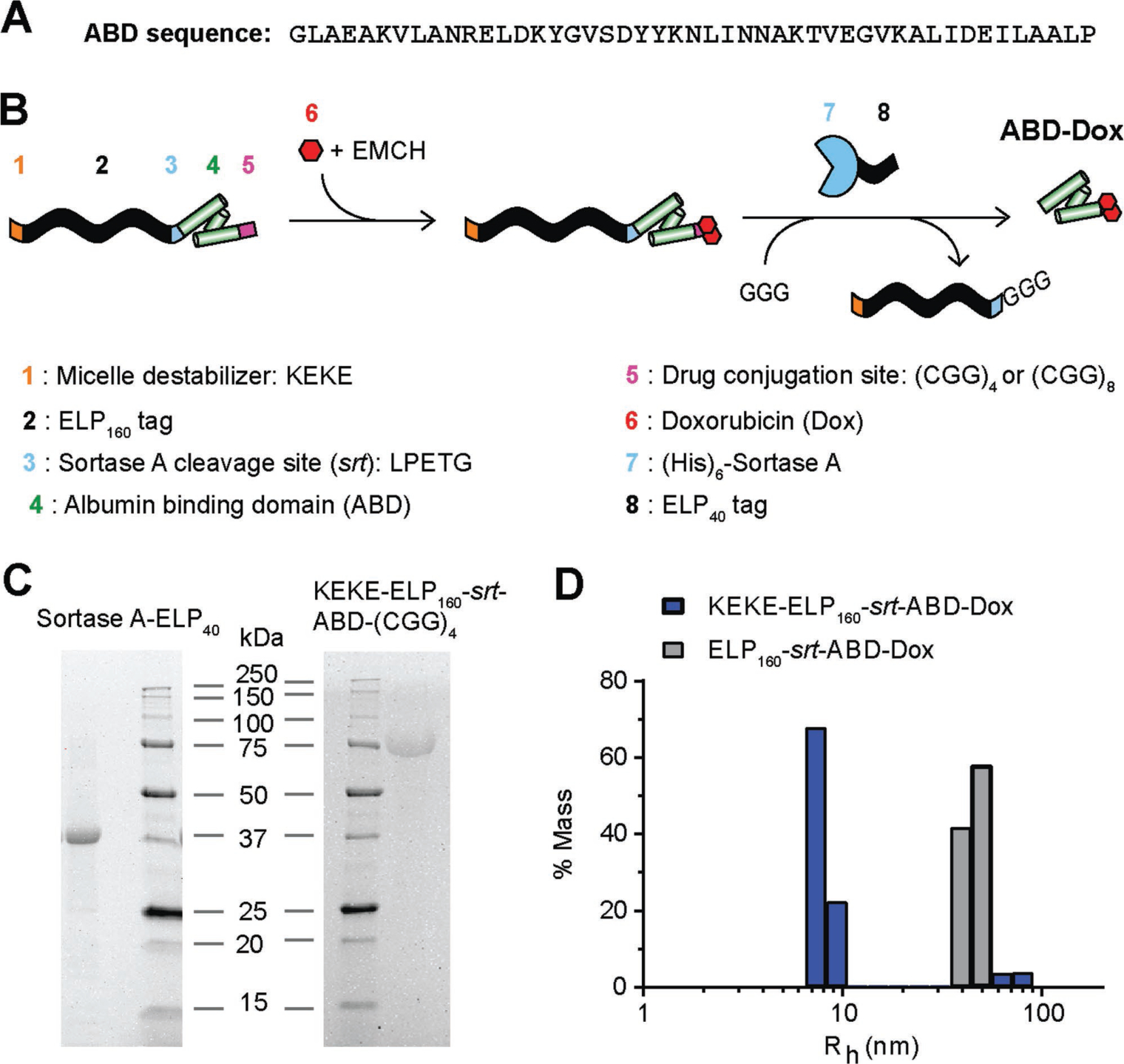
ABD–Dox synthesis. A) Amino acid sequence of ABD. B) Schematic illustration of the design and synthesis steps. ELP was used as a purification tag for ABD purification from bacteria and was removed following drug conjugation using sortase A. The KEKE peptide was included at the N-terminus to disrupt micelle self-assembly upon Dox conjugation, and to enable the subsequent sortase A cleavage of the ELP from the ABD–Dox conjugate. C) SDS-PAGE analysis confirmed successful purification of KEKE–ELP160–srt–ABD–(GGC)4 and (His)6–sortase A–ELP40 with theoretical molecular weights of 69.4 and 34.4 kDa, respectively. D) Hydrodynamic radius of ELP160–srt–ABD–Dox conjugates was measured with DLS. ELP160–srt–ABD–Dox conjugates without N-terminal KEKE segment self-assembled into micelles with Rh of 40–50 nm, whereas conjugates containing the N-terminal KEKE segment were mostly (≈90%) unimers with Rh < 10 nm.
