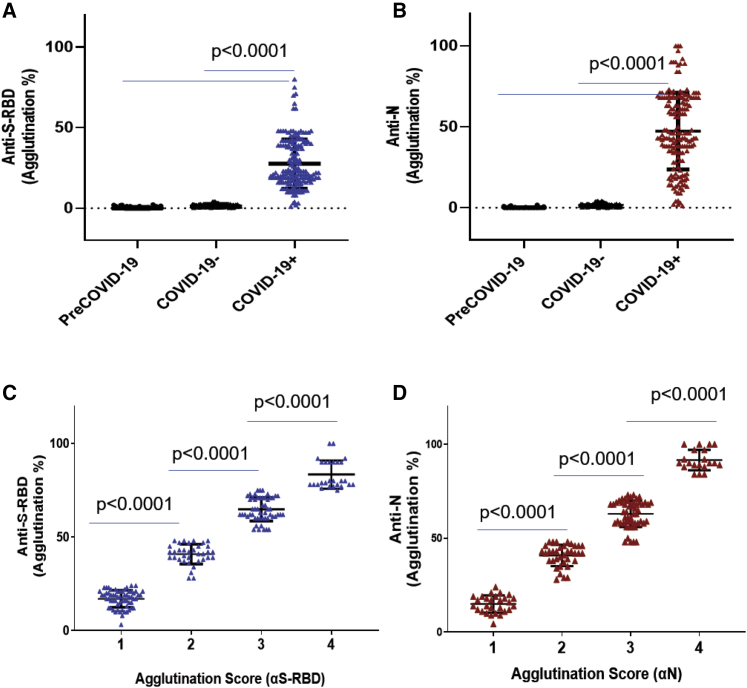
Figure 3.
Agglutination assay distinguished COVID-19+ from COVID-19− samples
(A and B) Comparison of S-RBD (A) and N antibody (B) responses between COVID-19+ (n = 169), COVID-19− (n = 121), and pre-COVID-19 (n = 100) samples determined by the aggregation assay.
(C and D) The strength of the S-RBD (C) and N antibody (D) response in the COVID-19+ (n = 169) plasma samples could be determined semi-quantitatively by the aggregation score (1–4 denotes weak to strong antibody response). Statistical analyses were performed using unpaired Student's t test with Welch's correction (p values shown on graph). Error bars represent standard deviation.