Introduction
Johnson & Johnson (J&J) received emergency use authorization from the Food and Drug Administration for its single-dose, adenovirus vector COVID-19 vaccine (Ad26.COV2.S) on February 27, 2021. As with the 2 mRNA vaccines that have also received emergency use authorization, local and systemic side effects were common and seen in over 50% of patients.1 No rashes other than injection-site reactions and delayed hypersensitivity reactions, now known as “COVID arm,” were reported, according to J&J's phase 3 interim analysis. Other than 5 reported cases of urticaria, there were no reports of “rash” or “dermatitis.”2
At present, there is a paucity of data on skin eruptions from the J&J COVID-19 vaccine. We report the case of an 83-yearold woman with a widespread erythematous annular eruption that resolved after a week of treatment with antihistamines and mid-potency topical steroids.
Case report
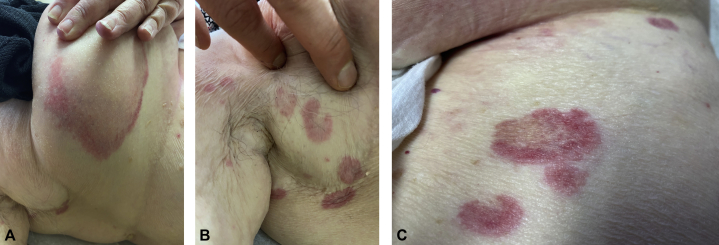
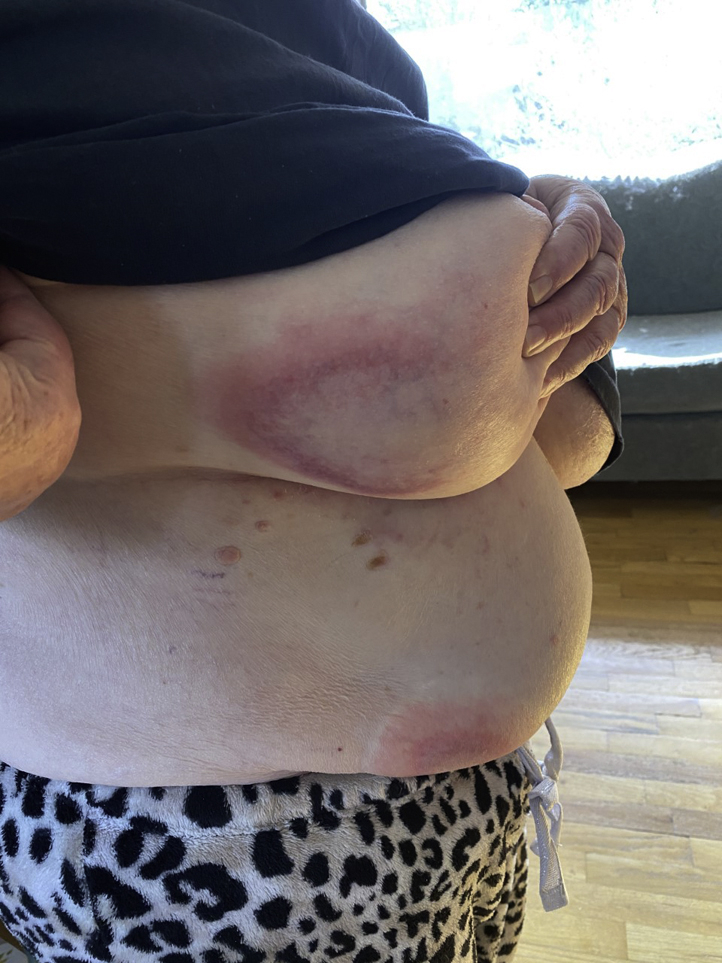
An 83-year-old woman received the J&J COVID-19 vaccine on March 12, 2021. Two days later, pruritic erythematous annular patches with central clearing started to appear on the breast, abdomen, and axilla (Fig 1). When she presented to our clinic 1 week later, she had more widespread disease, and her previous sites of involvement had developed scattered petechiae (Fig 2). She denied any oral or genital involvement and she otherwise felt well. She denied any prior history of COVID-19, and her review of systems was negative for fever, chills, body aches, sore throat, and diarrhea. Her past medical history included hypertension, hypothyroidism, and breast cancer. There were no recent changes in her medications, which included palbociclib, letrozole, and vitamin D. She did not use any over-the-counter medications or herbal supplements. The patient denied any history of persistent skin rash, allergies, or autoimmune disease.
Fig 1.
Two days after administration of the J&J COVID-19 vaccine.
Fig 2.
One week after administration of the J&J COVID-19 vaccine.
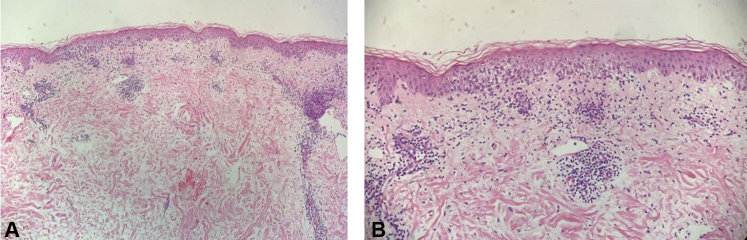
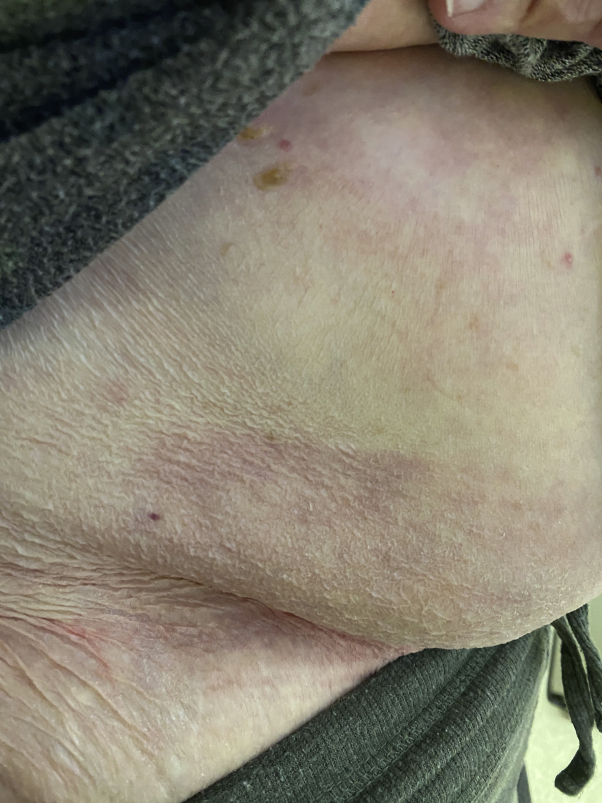
A representative lesion was biopsied and showed a mixed spongiotic and interface dermatitis with rare eosinophils and extravasated erythrocytes (Fig 3). The patient was started on fexofenadine 360 mg daily, cetirizine 20 mg nightly, and triamcinolone 0.1% cream twice daily. One week later, the patient reported that she was no longer getting new lesions and her pruritus had nearly resolved. At her 2-week follow-up, the patient was completely clear except for some residual postinflammatory erythema, and all treatment was discontinued (Fig 4).
Fig 3.
A, Low magnification and B, high magnification. The stratum corneum has basket-weave keratin. The epidermis has spongiosis and focal interface vacuolar changes. Eosinophil tagging is not seen at the dermoepidermal junction. There is moderate edema in the papillary dermis. There is a moderate superficial perivascular and interstitial infiltrate of lymphocytes with a small number of eosinophils and a few extravasated erythrocytes.
Fig 4.
Two-week follow-up after treatment with antihistamines and mid-potency topical steroids.
Discussion
The cutaneous manifestations of COVID-19 have been well documented and include morbilliform, urticarial, petechial, varicelliform, vasculopathic, and chilblain-like eruptions.3 Less is known about cutaneous adverse reactions to the COVID-19 vaccine; most information comes from anecdotal reports. We hope that our case will contribute to the limited clinical data on cutaneous adverse events from the COVID-19 vaccines. We strongly encourage all clinicians to report any adverse events from the COVID-19 vaccines so that we can better counsel and care for our patients.4 In conclusion, we report a case of a widespread annular eruption that developed 48 hours after administration of the J&J COVID-19 vaccine and that resolved with treatment with antihistamines and mid-potency topical steroids.
Conflicts of interest
Dr Song has been a consultant, speaker, or investigator for the following companies: AbbVie, Janssen, Amgen, Lilly, SUN pharma, Union Chimique Belge, Incyte, Novartis, Sanofi and Regeneron, and Castle Biosciences. Author Wong has no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Local reactions, systemic reactions, adverse events, and serious adverse events: Janssen COVID-19 Vaccine (J&J) | CDC. https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html Accessed April 3, 2021. Available at:
- 2.Biotech J. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document—FDA; 2021.
- 3.Suchonwanit P., Leerunyakul K., Kositkuljorn C. Cutaneous manifestations in COVID-19: lessons learned from current evidence. J Am Dermatol. 2020;83(1):e57–e60. doi: 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccine Adverse Event Reporting System (VAERS). Report an adverse event. https://vaers.hhs.gov/reportevent.html Accessed April 3, 2021. Available at: