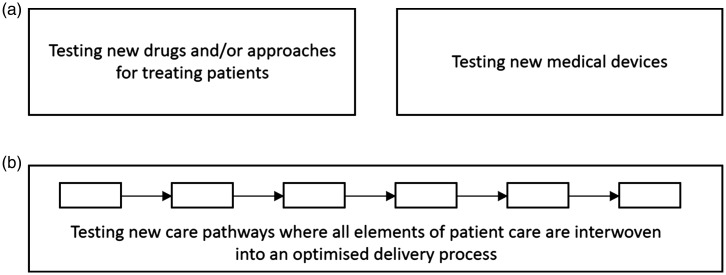
Figure 1.
Established and new methodology fields in the clinical trial landscape. (a) Established fields, CTIMPs or medical device studies, each normally focused on assessment of a single intervention (drug or device), or combined intervention (drug and device). (b) New field of clinical research, assessing the patient benefits from innovation in an integrated care pathway. Fills the gap that currently exists between CTIMP and medical device studies and supports subsequent uptake and adoption of the approach - the so called “Technology clinical trial”. CTIMP: Clinical trials of investigational medicinal product.